
CME Information
This activity is jointly provided by Partners for Advancing Clinical Education (Partners) and MedliveCME, LLC (previously known as PlatformQ Health Education, LLC) in collaboration with the Asthma and Allergy Foundation of America (AAFA), Kids with Food Allergies, a division of the Asthma and Allergy Foundation of America, and Food Allergy & Anaphylaxis Connection Team (FAACT).
Acknowledgement
This activity is supported by an educational grant from Genentech, a member of the Roche Group.
Credits Available
- Physicians — maximum of 0.75 AMA PRA Category 1 Credit(s)™
All other healthcare professionals completing this course will be issued a statement of participation.
Date of Release/Expiration
This activity was released on December 4, 2025, and is valid until December 4, 2026. Requests for credit must be made no later than December 4, 2026.
Target Audience
The educational design of this activity addresses the needs of Pediatrics and Primary Care Clinicians, and Allergy/Immunology Clinicians.
Program Overview
With recent FDA approvals of emerging treatments for food allergy (FA), including omalizumab and epinephrine nasal spray, clinicians must be up to date on these breakthrough therapies and evolving guidelines for FA. An expert allergy/immunology clinician and a primary care clinician will discuss essential updates on diagnosing IgE-mediated FA, selecting appropriate treatment options, and recognizing when to refer to specialists. The panel will also discuss the importance of decision-making and how it can help explain management approaches and treatment expectations, helping ensure the patient adheres to treatment. Discussions will be framed by pre-recorded patient vignettes to highlight the patient experience with FA.
Learning Objectives
After completing this activity, the participant should be better able to:
- Summarize the efficacy, safety for, and place in management of FA of targeted biologics and epinephrine nasal spray
- Review latest recommendations for the management of IgE-mediated FA and anaphylactic reaction
- Explain how to apply shared decision-making strategies to counsel patients and their caregivers about all their management options for IgE-mediated FA and anaphylactic reactions and to manage treatment expectation and improve safety
- Recognize patients who would benefit from referral to an allergy specialist for further management
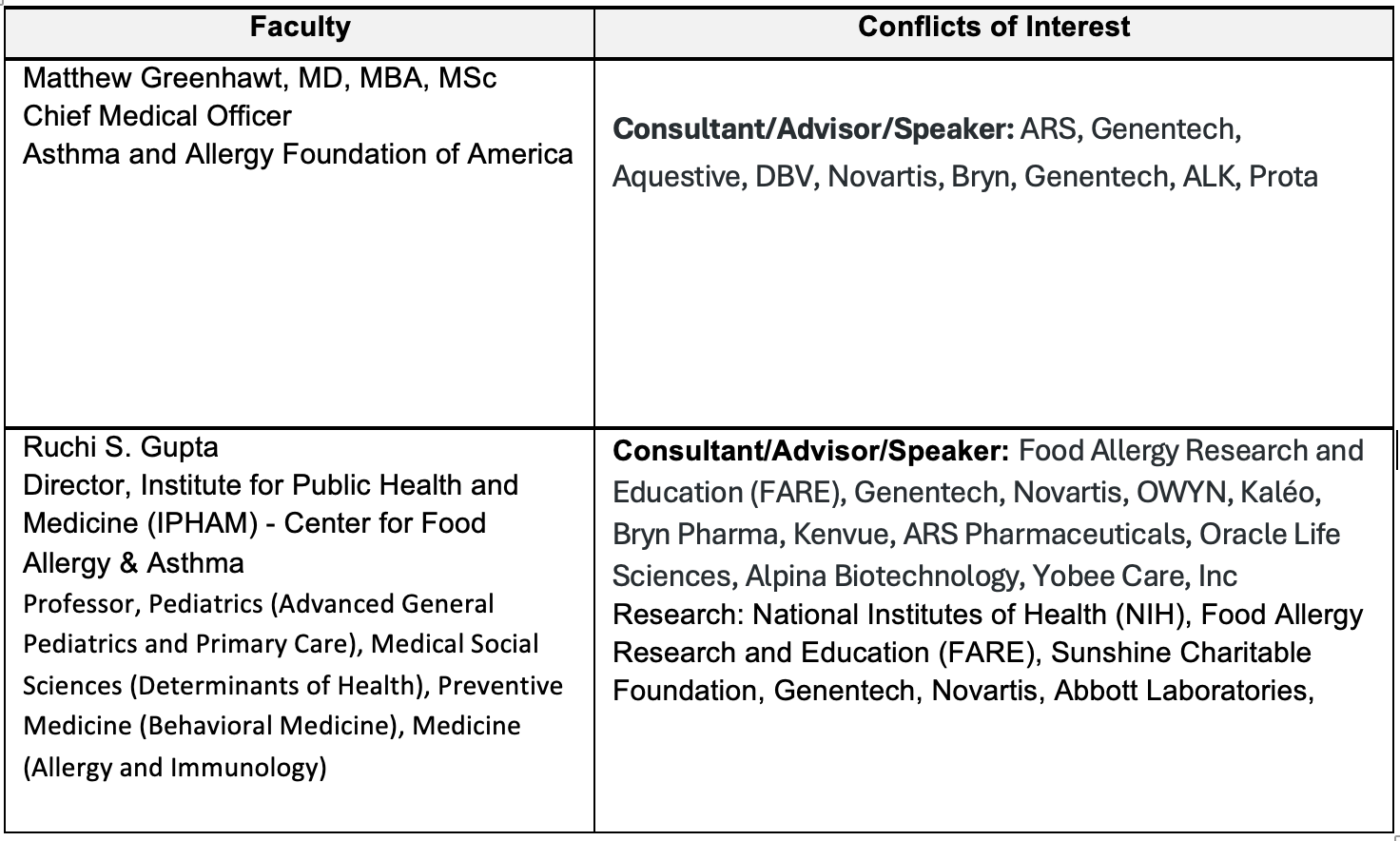
Faculty
Matthew Greenhawt, MD, MBA, MSc
Chief Medical Officer
Asthma and Allergy Foundation of America
Ruchi S. Gupta
Director, Institute for Public Health and Medicine (IPHAM) - Center for Food Allergy & Asthma
Professor, Pediatrics (Advanced General Pediatrics and Primary Care), Medical Social Sciences (Determinants of Health), Preventive Medicine (Behavioral Medicine), Medicine (Allergy and Immunology)
Joint Accreditation Statement

In support of improving patient care, this activity has been planned and implemented by Partners for Advancing Clinical Education (Partners) and MedliveCME, LLC (previously known as PlatformQ Health Education, LLC). Partners is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Physician Continuing Education
Partners designates this enduring material for a maximum of .75 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Estimated Time To Complete
This activity should take approximately 45 minutes to complete.
Instructions to Receive Credit
In order to receive credit for this activity, the participant must (1) read the target audience, learning objectives, and disclosure statements, (2) complete the educational activity online, and (3) complete the post-test and activity evaluation. To receive AMA PRA Category 1 Credits™, participants must receive a score of 75% on the post-test.
Hardware/Software Requirements
Participants will need a computer with a recent version of Adobe Flash installed, as well as an internet connection sufficient for streaming media.
Faculty and Disclosure of Conflicts of Interest
Partners for Advancing Clinical Education (Partners) requires every individual in a position to control educational content to disclose all financial relationships with ineligible companies that have occurred within the past 24 months. Ineligible companies are organizations whose primary business is producing, marketing, selling, re-selling, or distributing healthcare products used by or on patients.
All relevant financial relationships for anyone with the ability to control the content of this educational activity are listed below and have been mitigated according to Partners policies. Others involved in the planning of this activity have no relevant financial relationships.
Disclosure of Unlabeled Use
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The planners of this activity do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of the planners. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer
Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient’s conditions and possible contraindications and/or dangers in use, review of any applicable manufacturer’s product information, and comparison with recommendations of other authorities.
Medlive Privacy Policy
Contact Information
Accreditation Support:
For additional information about the accreditation of this activity, please visit https://partnersed.com.
Technical Support:
For any technical issues or issues with your CME Certificate, please contact MedLive at 877-394-1306 or at [email protected].
