Teen Pregnancies in the ED Part 2: Handling Complications
Author
Daniel Migliaccio, MD, FPD, FAAEM
Clinical Associate Professor, Division Director of Emergency Ultrasound, Ultrasound Fellowship Director, Department of Emergency Medicine, University of North Carolina at Chapel Hill
Peer Reviewer
Katherine Baranowski, MD, FAAP, FACEP
Chief, Division of Pediatric Emergency Medicine, Department of Emergency Medicine, New Jersey Medical School, Rutgers, The State University of New Jersey, Newark
Executive Summary
- Trauma is the No. 1 cause of nonobstetrical, pregnancy-associated maternal death in the United States.
- At ≥ 20 weeks’ gestation, the inferior vena cava can be compressed by a gravid uterus while the mother is lying supine. That can decrease venous return and cardiac output, leading to hypotension. This can be improved by positioning the mother in the left lateral decubitus position whenever possible.
- The diaphragm can elevate by up to 4 cm during pregnancy. This is important to keep in mind during chest tube insertion.
- As soon as maternal resuscitation allows, all pregnant women at ≥ 20 weeks’ gestation presenting with trauma should have cardiotocographic and fetal heart rate monitoring started and continued for a minimal period of four to six hours, even if the patient is asymptomatic.
- Regarding other trauma imaging, imaging should be obtained based on traumatic indications and should not be withheld simply due to pregnancy. Indications for emergent laparotomy or other trauma surgery monitoring/interventions should remain the same as in nonpregnant patients.
- By definition, gestational hypertension is a new onset of elevated blood pressure (systolic pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) after 20 weeks’ gestation without proteinuria or other signs of end-organ damage.
- Postpartum hemorrhage occurs in approximately 4% to 6% of all pregnancies, with uterine atony the most common etiology. Postpartum hemorrhage is defined as blood loss of 1,000 mL or more accompanied by signs or symptoms of hypovolemia (e.g., lightheadedness, tachycardia, palpitations, hypotension) within 24 hours of giving birth.
- If the exam is consistent with uterine atony, or no other clear source of traumatic etiology is identified, the next step is to administer oxytocin (Pitocin), either IV or IM, and perform bimanual uterine massage.
Introduction
Despite recent decline in teen birth rates in the United States, adolescent pregnancy rates remain high when compared to other developed countries.1,2 There are many unique barriers and considerations for the emergency care for adolescents who are pregnant, many of which are discussed in depth in “Teen Pregnancies in the ED Part 1,” including the most common emergency department (ED) presentations. This article will focus on the medical adverse outcomes and obstetrical complications that can occur in adolescent pregnancy. Topics include trauma in pregnancy; preeclampsia and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome; venous thromboembolism; as well as precipitous delivery and postpartum hemorrhage.
Trauma in Pregnancy
A 16-year-old female, gravidity 1, parity 0, at 33 weeks’ gestation arrives at the emergency department via emergency medical services after being involved in a motor vehicle crash. The patient was the restrained driver of a vehicle that was rear-ended at approximately 20 miles per hour. She had no loss of consciousness, self-extricated, and was ambulatory on the scene. Her vital signs are within normal limits. She is complaining of mild abdominal pain and cramping without vaginal bleeding or leakage of fluid. She reports normal fetal movement.
Trauma is the No. 1 cause of nonobstetrical, pregnancy-associated maternal death in the United States.3 The most common causes of trauma include motor vehicle collisions (accounting for 50% of pregnancy-related trauma), falls, and assault.4 Complications of trauma during pregnancy include preterm labor, premature rupture of membranes, placental abruption, fetal maternal hemorrhage, uterine rupture, and fetal demise.
It is important to note that some of these complications, particularly placental abruption, can occur with even minor maternal injuries.5 During evaluation and resuscitation of a pregnant trauma patient, maternal resuscitation always takes priority because fetal survival is dependent on maternal survival. Fetal viability is considered at approximately 22-24 weeks’ gestation (fundal height at least at or above the umbilicus).
The initial approach to a pregnant trauma patient should follow the Advanced Trauma Life Support (ATLS) guidelines. However, there are additional considerations in the evaluation and treatment of pregnant women presenting with trauma. Unique anatomic and physiologic changes of pregnancy can affect trauma resuscitation and care.
- Pregnancy results in a relative hypervolemic state with an increase in cardiac output of 30% to 50%. Therefore, clinically significant injuries can be easily masked. With this, uterine injury can result in significant maternal hemorrhage, given the substantial increase in uterine blood flow during pregnancy.6
- In addition, pregnant trauma patients can develop a condition called supine hypotension syndrome. At ≥ 20 weeks’ gestation, the inferior vena cava can be compressed by a gravid uterus while the mother is lying supine. That can decrease venous return and cardiac output, leading to hypotension. This can be improved by positioning the mother in the left lateral decubitus position whenever possible.6
- The diaphragm can elevate by up to 4 cm during pregnancy. This is important to keep in mind during chest tube insertion. Additional physiologic respiratory changes during pregnancy include a decrease in functional residual volume and in partial pressure of carbon dioxide, making respiratory compensation more difficult. It is important to keep in mind that if intubation is indicated, pregnant women often will become hypoxic more quickly during the apneic period.6 They typically also are at higher risk of aspiration given delayed gastric emptying and upward displacement of intra-abdominal organs during pregnancy.
After a standard primary survey per the ATLS protocol, unique adjuncts of a secondary trauma survey in a pregnant patient include an assessment of fetal heart tones and uterine size, as well as a thorough abdominal and genitourinary examination to assess for uterine tenderness, ecchymosis, and vaginal bleeding. As soon as maternal resuscitation allows, all pregnant women at ≥ 20 weeks’ gestation presenting with trauma should have cardiotocographic and fetal heart rate monitoring started and continued for a minimal period of four to six hours, even if the patient is asymptomatic.5,7 If there are any signs of fetal distress (late decelerations, persistent tachycardia, or bradycardia), admission for monitoring for a minimum of 24 hours is indicated. In discussion with an obstetrician, emergent delivery may need to be considered if the fetal distress persists. Additionally, an obstetrical ultrasound should be performed to assess for fetal size/gestational age, fetal cardiac activity, and fetal movement and for evidence of uterine rupture or placental abruption.
Regarding other trauma imaging, imaging should be obtained based on traumatic indications and should not be withheld simply due to pregnancy. The risk of missed or delayed diagnosis of traumatic injury outweighs the risk of fetal exposure to ionizing radiation.8 Indications for emergent laparotomy or other trauma surgery monitoring/interventions should remain the same as in nonpregnant patients.
Other therapeutic considerations should include tetanus, diphtheria, and pertussis prophylaxis if there has been no booster in the past five years, as well as administration of Rho(D) immunoglobulin (Rhogam) in all Rhesus-negative patients.9
Traumatic Placental Abruption
Placental abruption can occur with even seemingly minor trauma in pregnant women ≥ 20 weeks’ gestation.10 This is in part because, with trauma, there is a sudden stretching of the underlying uterine wall that can cause shearing stress on an inelastic placenta. Continuous fetal monitoring for signs of uterine irritability (three or more contractions in one hour) or fetal distress is essential. Note that although placental abruption can be diagnosed via obstetrical ultrasound, the sensitivity for this method is exceedingly low (25%), making it an unreliable test to rule out placental abruption in trauma.11
Typical symptoms of placental abruption include uterine contractions or back pain with vaginal bleeding. The uterus often is firm, and contractions typically are high-frequency but low-amplitude.12 Retrouterine placental abruption may mask blood loss because blood can be contained behind the uterus, and some patients with abruption will have no vaginal bleeding at all. Placental abruptions result in significantly increased morbidity and mortality for both the mother and the fetus via an increased risk of maternal disseminated intravascular coagulation (DIC) and fetal-maternal hemorrhage.13
If there is concern for a severe traumatic mechanism, DIC laboratory tests (coagulation profile with fibrinogen) and a Kleihauer-Betke test (quantifies the presence of fetal hemoglobin in maternal circulation) could be obtained. If there are concerns for maternal or fetal compromise, an obstetrical evaluation is emergently indicated, as delivery may be imminent.
Traumatic Arrest and Perimortem Cesarean Delivery
A perimortem cesarean delivery is defined as a cesarean delivery performed during active or imminent maternal cardiac arrest. The primary goal is to resuscitate the mother, while the secondary goal is to potentially improve fetal viability. Although the traditional indication for perimortum cesarean delivery was a gestational age of 24 weeks or older, most recommendations now state that it should be considered in any patient whose uterine fundus can be palpated at the umbilicus or above, with the presumption that this makes the gestational age at least 20 weeks.14
In addition to gestational age, a perimortem cesarean delivery should be performed within four minutes of maternal arrest.15 Although not a comprehensive description, the following are some key features of the perimortem cesarean delivery technique:6
- The physician performing the procedure should be the physician with the most surgical experience whenever possible.
- Using a No. 10 blade, an initial incision should be made beginning from the xiphoid process (or at least from the level of the uterine fundus) and extending inferiorly to the pubic symphysis.
- Once through the subcutaneous tissue to the peritoneum, an incision through the peritoneum should be made using either a scalpel or scissors to deliver the uterus.
- Then, a midline vertical incision should be made on the lower portion of the uterus while taking caution to avoid the placenta, bowel, and bladder.
- Once in the uterine cavity, the physician should digitally separate the uterine wall away from the fetus. Then, the incision can be extended with scissors superiorly until the baby is exposed.
- The cord then should be clamped and cut, and infant resuscitation should begin.
- Simultaneously, the uterine and abdominal cavity should be packed to limit further bleeding while continued maternal resuscitation efforts are made.
- If return of spontaneous circulation is achieved, further surgical closure should be continued in the operating room, and the mother should receive broad-spectrum prophylactic antibiotics.
Preeclampsia, Eclampsia and HELLP Syndrome
A 16-year-old female, gravidity 1, parity 0, at 32 weeks’ gestation presents to the emergency department with a headache for the past two days. The headache is diffuse and has progressively worsened. It is associated with blurred vision. She denies any fevers, vomiting, or head trauma as well as any contractions, vaginal bleeding, or leakage of fluid. Her vital signs are temperature 36.8°C, heart rate 107 beats/minute, blood pressure 165/95 mmHg, and respiratory rate 22 breaths/minute.
Hypertensive disorders complicate approximately 10% of pregnancies worldwide.16 By definition, gestational hypertension is a new onset of elevated blood pressure (systolic pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) after 20 weeks’ gestation without proteinuria or other signs of end-organ damage.17 This patient would meet criteria for severe gestational hypertension, which is defined as a systolic pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 110 mmHg. In a newly hypertensive pregnant patient, a key goal in the initial evaluation is to determine the correct diagnosis: gestational hypertension, severe gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome. Each of these diagnoses involves a different course of management and prognosis. See Table 1 for terminology definitions.
Table 1. Terminology: Hypertensive Disorders of Pregnancy19,20 | |
| Term | Definition |
Chronic hypertension |
|
Gestational hypertension |
|
Severe gestational hypertension |
|
Preeclampsia |
|
Preeclampsia with severe features |
|
Eclampsia |
|
Hemolysis, elevated liver enzymes, and low platelet count (HELLP) |
|
Some of these conditions are considered to exist on a spectrum and often can progress to a more severe condition over time. For example, 10% to 50% of women diagnosed with gestational hypertension will go on to develop preeclampsia within a few weeks of initial diagnosis. Then, preeclampsia with severe features can develop rapidly over a period of days.18 It is important for emergency department providers to be able to distinguish between chronic hypertension, gestational hypertension, preeclampsia without severe features, where patients can be discharged with close follow-up and outpatient management, vs. preeclampsia with severe features, eclampsia, and HELLP, which require emergent actions.
ED Workup for a Hypertensive Pregnant Patient
If the patient is otherwise stable (i.e., no seizure-like activity, respiratory distress, altered mental status, hemodynamic instability, etc.), the initial step is to obtain a thorough history, including gravidity and parity numbers, prior pregnancy complications, previous medical history and medications, gestational age, and presence of symptoms. Specifically, it is important to ask whether the patient has had any symptoms that would be concerning for the diagnosis of preeclampsia with severe features. (See Table 2.)
Table 2. Severe Features Associated with Preeclampsia17 |
Systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 110 mmHg and proteinuria (with or without signs and symptoms of end-organ damage) |
New-onset cerebral or visual disturbance
|
Severe, persistent right upper quadrant or epigastric abdominal pain not accounted for by alternative diagnosis |
Serum transaminase ≥ two times the upper limit of normal |
Platelet count < 100,000 platelets per microliter |
Renal insufficiency (creatinine > 1.1 mg/dL) |
Pulmonary edema |
On physical examination, providers should assess for crackles, bilateral lower extremity edema, or signs of respiratory distress that would be concerning for a fluid overload status with pulmonary edema. The examination also should include a neurologic examination, including reflexes, and an abdominal examination to assess for right upper quadrant (RUQ) tenderness.
Laboratory workup includes complete blood count (to assess for anemia or thrombocytopenia), basic metabolic panel (for serum creatinine), liver function tests (to assess for transaminitis), urinalysis (to assess for proteinuria), urine protein/creatinine ratio, uric acid, and lactate dehydrogenase (elevated levels are associated with adverse maternal and fetal outcomes).21,22
See Table 3 for a summary of how to treat and manage various pregnancy-related hypertensive disorders.
Table 3. Emergency Department Management of Hypertensive Disorders of Pregnancy22-26 | |
| Hypertensive Disorder | Treatment |
Preeclampsia without severe features |
|
Preeclampsia with severe features |
|
Eclampsia |
|
HELLP syndrome |
|
Key: OB: obstetrical/obstetrician; MgSO4: magnesium sulfate; IV: intravenous; BP: blood pressure; ABCs: airway, breathing, circulation; RSI: rapid sequence intubation; HELLP: hemolysis, elevated liver enzymes, and low platelet count | |
Venous Thromboembolism in Pregnancy
A 17-year-old female, gravidity 1, parity 0, at 34 weeks’ gestation presents with sudden-onset, sharp, left-sided chest pain and shortness of breath beginning five hours prior to arrival. She states that she has had intermittent dyspnea that has worsened gradually over the past few weeks and more acutely today. She has had no abdominal pain, contractions, or vaginal bleeding. Vital signs are temperature 37.4°C, heart rate 116 beats/minute, blood pressure 110/78 mmHg, and respiratory rate 24 breaths/minute.
Pulmonary embolism (PE) is the leading cause of maternal death in the United States.27 The occurrence of venous thromboembolism (VTE) in pregnancy is 10 times that of the general population. Evaluation of VTE in pregnancy is further complicated by the fact that symptoms concerning for VTE also are often common signs and symptoms of normal pregnancy (e.g., dyspnea, tachycardia, and/or lower extremity swelling).28 Additionally, traditional diagnostic testing for pulmonary embolism poses higher risks to the fetus, which further complicates the VTE workup in pregnancy.
Pregnant women meet all three of Virchow’s triad for VTE, including hypercoagulation, vascular damage, and venous stasis.29 Pregnancy-specific VTE risk factors include grand multiparity, age > 35 years, obesity, hyperemesis, bed rest for more than four days, and preeclampsia.30 It is important to note that women remain at higher risk of VTE for more than eight weeks postpartum. Signs and symptoms of VTE are similar in pregnant and nonpregnant individuals. For deep vein thrombosis (DVT), one would expect to see asymmetric extremity swelling (typically lower extremities) and pain. Of note, the majority of DVTs are left-sided (90%).31 For PE, symptoms vary from mild dyspnea and tachypnea to full cardiopulmonary collapse.32
Figure 1 shows an approach to the diagnosis of VTE in pregnant patients. On physical exam, a neurovascular exam should be performed of the lower extremity and measurements should be taken for both legs to determine if there is any discrepancy.
Figure 1. Diagnostic Approach to Venous Thromboembolism in Pregnancy |
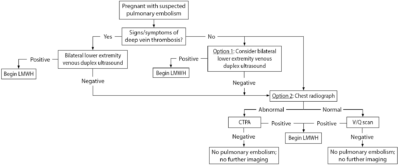 |
Key: LMWH: low molecular-weight heparin; CTPA: computed tomography pulmonary angiogram; V/Q: ventilation perfusion |
If there is clinical concern for DVT, clinicians should obtain compression duplex ultrasounds of the lower extremities. This diagnostic test is noninvasive, posing little risk to the fetus without exposure to radiation, and has a sensitivity of 89% to 96%.33,34 If the presentation is concerning for PE, some would recommend starting with bilateral lower extremity compression duplex ultrasounds, even in patients without the clinical signs of DVT. Given that treatment would be the same for both conditions, clinicians should stop further diagnostic workup if duplex ultrasounds are positive for DVT, therefore minimizing radiation exposure to the mother and fetus.34
In nonpregnant women, a D-dimer often is used as a screening test for patients who are otherwise at low risk for VTE. However, D-dimer levels are elevated in normal pregnancies and gradually increase with gestational age and into the postpartum period. Therefore, they are not as reliable as screening tools.
A 2019 study by Van der Pol et al proposed the YEARS algorithm, which used three criteria (clinical signs of DVT, hemoptysis, and PE as the most likely diagnosis) plus a D-dimer for the diagnostic evaluation of PE in pregnant women.35 The authors concluded that PE was safely ruled out by the pregnancy-adapted YEARS algorithm and that chest computed tomography (CT) was avoided in a large cohort of patients (32% to 65%).
However, external validation of this study is still needed. Therefore, use of D-dimer for diagnostic evaluation of PE in pregnancy remains controversial. A chest X-ray can be obtained prior to more invasive or high radiation exposing tests, since it can rule out other pathologies (e.g., pneumonia, pneumothorax), and, if abnormal, may increase clinical suspicion for PE.
Abnormal features that could be attributed to PE include atelectasis, pulmonary edema, pleural effusions, and focal opacities.36 The decision to proceed with a CT pulmonary angiogram (CTPA), a ventilation perfusion (V/Q) scan, or a magnetic resonance angiogram (MRA) is multifactorial, including availability, institutional guidelines, discussions with the radiologist, and shared decision-making with the patient. However, studies have shown that CTPA typically is the diagnostic study of choice in patients who have abnormal findings on chest radiography.37
It is important to note that both CTPA and V/Q scans carry similar fetal radiation exposure risks (~0.5 mGy) and that one study exposes a fetus to well below the threshold for fetal malformation related to radiation (threshold 100 mGy to 200 mGy).38
Treatment of VTE in pregnancy should begin with stabilization (airway, breathing, and circulation) and determination of whether the patient has cardiovascular compromise severe enough to be life-threatening, requiring potential thrombolytic therapy or percutaneous or surgical intervention.
If acutely stable, therapeutic anticoagulation is indicated, with low molecular-weight heparin (LMWH) (e.g., enoxaparin) being the first-line medication.34,39,40 Therapeutic anticoagulation should be continued for a minimum of three to six months and at least until six weeks postpartum.34
Precipitous Delivery and Postpartum Hemorrhage
A 15-year-old female, gravidity 1, parity 0, at 38 weeks’ gestation presents by emergency medical services with strong uterine contractions reported to be two to three minutes apart. The patient is in obvious distress and states that she feels like she needs to push. On genitourinary examination, the infant’s head is visible at the vaginal introitus.
Emergency medicine clinicians also should be prepared in the case of a precipitous vaginal delivery. Precipitous labor is defined as labor that lasts less than three hours from onset of contractions to completion of delivery.41 Women presenting in precipitous labor typically have had little or no prenatal care and therefore the neonates are more likely to be premature or have higher-risk features.42
When a woman presents in precipitous labor, if delivery is not immediately imminent, clinicians should obtain a brief history, including questions regarding gestational age, number of babies expected to be delivered, when contractions began and how far apart they are, leakage of fluid from the vagina, fetal movement, any complications with the current or past pregnancies, and whether the patient has had regular prenatal care. While there are numerous possible maternal or fetal complications that can occur, the vast majority of precipitous deliveries presenting to the ED occur without complication. While all peripartum complications cannot be discussed in detail here, further details regarding postpartum hemorrhage (one of the most common and life-threatening complications of childbirth) are described later.
Uncomplicated Delivery of the Newborn
Physical delivery of the newborn as the baby passes through a completely dilated cervix through the birth canal is known as the second stage of labor. (See Figure 2.) During this time, it is the delivering clinician’s responsibility to reduce the risks of maternal trauma and neonatal injury. Multiple methods that are subtly different can be used to deliver the head of the baby. There is no true consensus regarding which method is best.43-47
Figure 2. Delivery Process |
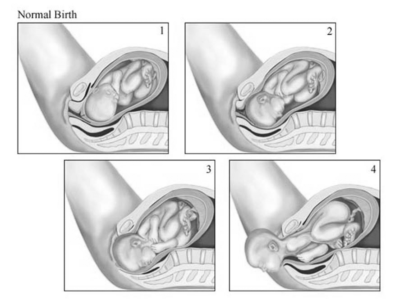 |
1. Fetal engagement begins 2. Further descent, fetal rotation begins 3. Fetal rotation completes, extension begins 4. Complete fetal extension Source: © Relias LLC 2024 |
One common method is the “hands on” approach, in which one hand is used to place gentle pressure on the occiput of the baby’s head through crowning while simultaneously using the thumb and index finger of the other hand to provide counter protection to the perineum.48,49
After the fetal head has been completely delivered, allow for restitution of the fetal head (i.e., rotation of the head to where the face is oriented laterally to the right or left). After restitution, the fetal shoulders will need to be delivered. The clinician should place one hand on each side of the head and should place gentle downward traction toward the mother’s sacrum to help guide the baby’s anterior shoulder under the maternal symphysis pubis. After delivery of the anterior shoulder, the posterior shoulder can be released by then applying direct, gentle, upward traction on the baby. Once the shoulders are delivered, the remainder of the neonate’s body will easily expulse. The cord then should be clamped and cut.
Finally, the third stage of labor includes delivery of the placenta. Active management of delivery of the placenta includes beginning a uterotonic agent, typically oxytocin intramuscularly (IM) or intravenously (IV). Then, the clinician should use one hand to apply firm, but controlled, downward traction on the transected umbilical cord while applying firm downward pressure suprapubically to prevent uterine inversion.50 After delivery of the placenta, a clinician should explore for lacerations that need repair and assess for uterine atony to help reduce the risk of postpartum hemorrhage.
Postpartum Hemorrhage
Postpartum hemorrhage occurs in approximately 4% to 6% of all pregnancies, with uterine atony the most common etiology.51 Postpartum hemorrhage is defined as blood loss of 1,000 mL or more accompanied by signs or symptoms of hypovolemia (e.g., lightheadedness, tachycardia, palpitations, hypotension) within 24 hours of giving birth.52 The initial management step for postpartum hemorrhage includes cardiopulmonary support and resuscitation of the mother. Fundamental to adequate resuscitation includes ensuring the mother is on a monitor and has adequate vascular access (a minimum of two large-bore IV infusions). Intravenous fluid provision can be initiated to maintain blood pressure.
However, for any patient with significant hemorrhage or hemodynamic instability, strong consideration should be given to early transfusion with blood products, beginning with packed red blood cells and consideration for a massive transfusion protocol with other blood products, such as platelets, fresh frozen plasma, and consideration of cryoprecipitate/adjunctive calcium provision. Perform an exam to identify the cause of the hemorrhage (e.g., boggy uterus suggestive of uterine atony vs. bleeding laceration from birth trauma). If the exam is consistent with uterine atony, or no other clear source of traumatic etiology is identified, the next step is to administer oxytocin (Pitocin), either IV or IM, and perform bimanual uterine massage.
A bimanual uterine massage is performed by placing one hand inside the vagina and placing firm pressure on the uterine body with a closed fist. The other hand is placed over the uterine fundus to compress it down against the uterine body. If oxytocin and uterine massage fail to control the hemorrhage, several additional medications can be considered. Table 4 describes options for medical management of postpartum hemorrhage, the typical recommended dosages, and special considerations regarding contraindications.51,53,54 Should the hemorrhage persist despite exhaustion of medical management, the uterus and vaginal canal should be packed, and emergent obstetrical consultation or transfer to a facility with obstetric specialists is indicated because these patients may require hysterectomy.
Table 4. Options for Medical Management of Postpartum Hemorrhage53-55 | |||
| Medication | Dose | Contraindications | Adverse Effects |
Oxytocin | 10 units IM or 10 to 40 units per 500-1,000 mL saline as a continuous infusion | Possible hypotension with IV use following cesarean delivery | Minimal; nausea or vomiting with prolonged use |
Methylergonovine | 0.2 mg IM taken orally every two to four hours | Hypertension or preeclampsia; known cardiovascular disease | Severe hypertension (especially if given intravenously), nausea, or vomiting |
Carboprost | 0.25 mg IM or into myometrium every 15-90 minutes; max cumulative dose 2 mg | Avoid in patients with asthma or significant renal, hepatic, or cardiac disease | Nausea or vomiting, diarrhea, transient fever, shivering, headache, bronchospasm |
Misoprostol (PGE1) | 1,000 mcg rectally once; alternate dose 400-800 mcg saline lock | Use caution in patients with cardiovascular disease | Nausea or vomiting, diarrhea, transient fever, shivering, headache |
Tranexamic acid (TXA) | 1 g infused in 10-20 minutes | Rare/none | Thrombosis/venous thromboembolism |
IM: intramuscularly; IV: intravenously | |||
Conclusion
Teen pregnancies are at higher risk of obstetrical complications with adverse maternal and fetal outcomes than adult pregnancies in the United States.55,56 Pediatric emergency medicine clinicians should be familiar with the diagnostic evaluation and management of common emergent obstetrical complications, including hypertensive emergencies, acute trauma, postpartum hemorrhage, and precipitous delivery.
References
- Centers for Disease Control and Prevention. Reproductive health: Teen pregnancy. Updated Nov. 15, 2021. https://www.cdc.gov/teenpregnancy/about/index.htm
- United Nations Statistics Division. Demographic Yearbook 2013. Published 2015. http://unstats.un.org/unsd/demographic/products/dyb/dyb2013/Table10.pdf
- Chang J, Berg C, Saltzman L, Herndon J. Homicide. A leading cause of injury deaths among pregnant and postpartum women in the United States, 1991-1999. Am J Public Health 2005;95:471.
- El-Kady D, Gilbert WM, Anderson J, et al. Trauma during pregnancy: An analysis of maternal and fetal outcomes in a large population. Am J Obstet Gynecol 2004;190:1661-1668.
- Pearlman MD, Tintinalli JE, Lorenz RP. Blunt trauma during pregnancy. N Engl J Med 1990;323:1609-1613.
- Barclay-Buchanan CJ, Barton MA. Trauma in pregnancy. In: Tintinalli JE, Ma O, Yealy DM eds. Tintinalli's Emergency Medicine: A Comprehensive Study Guide. 9th ed. McGraw-Hill;2019:1680-1682.
- Barraco RD, Chiu WC, Clancy TV, et al. Practice management guidelines for the diagnosis and treatment of injury in the pregnancy patient: The EAST practice management guidelines work group. J Trauma 2010;69:211-214.
- Sadro C, Bernstein MP, Kanal KM. Imaging of trauma: Part 2, abdominal trauma and pregnancy — a radiologist’s guide to doing what is best for the mother and baby. Am J Roentgenol 2012;199:1207-1219.
- [No authors listed]. ACOG educational bulletin. Obstetric aspects of trauma management. Number 251, September 1998 (replaces Number 151, January 1991, and Number 161, November 1991). American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 1999;64:87-94.
- Cheng HT, Wang YC, Lo HC, et al. Trauma during pregnancy: A population-based analysis of maternal outcome. World J Surg 2012;36:2767-2775.
- Glantz C, Purnell L. Clinical utility of sonography in the diagnosis and treatment of placental abruption. J Ultrasound Med 2002;21:837-840.
- Kasai M, Aoki S, Ogawa M, et al. Prediction of perinatal outcomes based on primary symptoms in women with placental abruption. J Obstet Gynaecol Res 2015;41:850-856.
- Solomonia N, Playforth K, Reynolds EW. Fetal-maternal hemorrhage: A case and literature review. AJP Rep 2012;2:7-14.
- Alexander AM, Sheraton M, Lobrano S. Perimortem cesarean delivery. Updated Sept. 12, 2022. In: StatPearls [Internet]. https://www.ncbi.nlm.nih.gov/books/NBK534240/
- Katz VL, Dotters DJ, Droegemueller W. Perimortem cesarean delivery. Obstet Gynecol 1986;68:571-576.
- Aronow WS. Hypertensive disorders in pregnancy. Ann Transl Med 2017;5:266.
- [No authors listed]. ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol 2019;133:1.
- Saudan P, Brown MA, Buddle ML, Jones M. Does gestational hypertension become pre-eclampsia? Br J Obstet Gynaecol 1998;105:117711-84.
- Hoffman SD, ed. Kids Having Kids: Economic Costs and Social Consequences of Teen Pregnancy. The Urban Institute Press; 2008.
- Assini-Meytin LC, Green KM. Long-term consequences of adolescent parenthood among African-American urban youth: A propensity score matching approach. J Adolesc Health 2015;56:529-535.
- Lam C, Lim KH, Kang D-H, Karumanchi SA. Uric acid and preeclampsia. Semin Nephrol 2005;25:56-60.
- Townsend R, O’Brien P, Khalil A. Current best practice in the management of hypertensive disorders in pregnancy. Integr Blood Press Control 2016;9:79-94.
- Wang B. Preeclampsia and eclampsia. CORE EM. https://coreem.net/core/preeclampsia-and-eclampsia/#background
- Houry DE, Salhi BA. Acute Complications of pregnancy. In: Marx, J, ed. Rosen’s Emergency Medicine. 8th ed. Elsevier Saunders;2014:2282-2302.
- Padden OM. HELLP syndrome: Recognition and perinatal management. Am Fam Physician 19991;60:829-836.
- Magann EF, Bass D, Chauhan SP, et al. Antepartum corticosteroids: Disease stabilization in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelets (HELLP). Am J Obstet Gynecol 1994;171:1148-1153.
- Chang J, Elam-Evans LD, Berg CJ, et al. Pregnancy-related mortality surveillance—United States, 1991-1999. MMWR Surveill Summ 2003;52:1-8.
- Pescatore R. D-Dimer and pregnancy: The DiPEP Study. REBEL EM. Published March 19, 2018. https://rebelem.com/d-dimer-and-pregnancy-the-dipep-study/
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: A 30-year population-based study. Ann Intern Med 2005;143:697-706.
- Zotz RB, Gerhardt A, Scharf RE. Prediction, prevention and treatment of venous thromboembolic disease in pregnancy. Semin Thromb Hemost 2003;29:143-154.
- Nelson-Piercy C. Handbook of Obstetric Medicine. 4th ed. Informa UK Ltd;2010.
- Dresang LT, Fontaine P, Leeman L, King VJ. Venous thromboembolism during pregnancy. Am Fam Physician 2008;77:1709-1716.
- Qaseem A, Snow V, Barry P, et al; Joint American Academy of Family Physicians/American College of Physicians Panel on Deep Venous Thrombosis/Pulmonary Embolism. Current diagnosis of venous thromboembolism in primary care: A clinical practice guideline from the American Academy of Family Physicians and the American College of Physicians. Ann Intern Med 2007;146:57-62.
- Greer IA, Thomson AJ. Thromboembolic disease in pregnancy and the puerperium: Acute management. Guidelines and Audit Committee of the Royal College of Obstetricians and Gynaecologists. Reviewed 2018. https://www.rcog.org.uk/media/wj2lpco5/gtg-37b-1.pdf
- Van der pol LM, Tromeur C, Bistervels IM, et. al. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med 2019;380:1139-1149.
- Fidler JL, Patz EF Jr, Ravin CE. Cardiopulmonary complications of pregnancy: radiographic findings. AJR Am J Roentgenol 1993;161:937-942.
- Daftary A, Gregory M, Daftary A, et al. Chest radiograph as a triage tool in the imaging-based diagnosis of pulmonary embolism. AJR Am J Roentgenol 2005;185:132-134.
- Eskandar OS, Eckford SD, Watkinson T. Safety of diagnostic imaging in pregnancy. Part 1: X-ray, nuclear medicine investigations, computed tomography and contrast media. Obstet Gynaecol 2010;12:71-78.
- Krivak TC, Zorn KK. Venous thromboembolism in obstetrics and gynecology. Obstet Gynecol 2007;109:761-777.
- Dong B, Jirong Y, Liu G, et al. Thrombolytic therapy for pulmonary embolism. Cochrane Database Syst Rev 2006;(2):CD004437.
- Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: Final data for 2018. Natl Vital Stat Rep 2019;68:1-47.
- Lee MO, Brown LL, Bender J, et al. A medical simulation-based educational intervention for emergency medicine residents in neonatal resuscitation. Acad Emerg Med 2012;19:577-585.
- Dahlen HG, Homer CS, Cooke M, et al. Perineal outcomes and maternal comfort related to the application of perineal warm packs in the second stage of labor: A randomized controlled trial. Birth 2007;34:282-290.
- Laine K, Pirhonen T, Rolland R, Pirhonen J. Decreasing the incidence of anal sphincter tears during delivery. Obstet Gynecol 2008;111:1053-1057.
- Pirhonen JP, Grenman SE, Haadem K, et al. Frequency of anal sphincter rupture at delivery in Sweden and Finland — result of difference in manual help to the baby’s head. Acta Obstet Gynecol Scand 1998;77:974-977.
- McCandlish R, Bowler U, van Asten H, et al. A randomised controlled trial of care of the perineum during second stage of normal labour. Br J Obstet Gynaecol 1998;105:1262-1272.
- Hals E, Øian P, Pirhonen T, et al. A multicenter interventional program to reduce the incidence of anal sphincter tears. Obstet Gynecol 2010;116:901-908.
- Kalis V, Jansova M, Rusavy Z. Perineal mapping. In: Ismail K, ed. Perineal Trauma at Childbirth. Springer;2016:41-70.
- Poulsen MØ, Madsen ML, Skriver-Moller A, Overgaard C. Does the Finnish intervention prevent obstetric anal sphincter injuries? A systematic review of the literature. BMJ Open 2015;5:e008346.
- Brandt ML. The mechanism and management of the third stage of labor. Am J Obstet Gynecol 1936;25:662-667.
- Dahlke JD, Bhalwal A, Chauhan SP. Obstetric emergencies: Shoulder dystocia and postpartum hemorrhage. Obstet Gynecol Clin North Am 2017;44:231-243.
- Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 183: Postpartum hemorrhage. Obstet Gynecol 2017;130:e168-e186.
- Mercado J, Brea I, Mendez B, et al. Critical obstetric and gynecologic procedures in the emergency department. Emerg Med Clin North Am 2013;31:207-236.
- McFarlin A. The emergency department management of precipitous delivery and neonatal resuscitation. Emerg Med Rep 2019;40:109-120.
- Magill MK, Wilcox R. Adolescent pregnancy and associated risks: Not just a result of maternal age. Am Fam Physician 2007;75:1310-1311.
- Ventura SJ, Hendershot GE. Infant health consequences of childbearing by teenagers and older mothers. Public Health Rep 1984;99:138-146.
