Preparing for Pediatric Asthma Exacerbations in the ED
Asthma is a common disease in pediatrics, with exacerbations occurring frequently. Every clinician who cares for children must be familiar with recognition and timely management to optimize each child's outcome.
Author
Daniel Migliaccio, MD, FPD, FAAEM, Clinical Associate Professor, Division Director of Emergency Ultrasound, Ultrasound Fellowship Director, Department of Emergency Medicine, University of North Carolina at Chapel Hill
Peer Reviewer
Steven M. Winograd, MD, FACEP, Attending Emergency Physician, Trinity Health Care, Samaritan, Troy, NY
Executive Summary
- Asthma is a complex and dynamic disease revolving around the concept of underlying inflammation, airway hyperresponsiveness, and airway obstruction that causes a variety of symptoms, including wheezing, shortness of breath, coughing, and chest tightness.
- In addition to asthma, other causes of small airway obstruction presenting with similar symptoms include viral bronchiolitis, bronchopulmonary dysplasia, cystic fibrosis, and congenital heart diseases.
- Unlike asthma, provision of bronchodilators and steroids does not affect the rate or duration of hospitalization in patients with bronchiolitis, who have no history of established asthma. Therefore, those medications typically are not indicated. Treatment for respiratory syncytial virus (RSV) primarily is supportive therapy (e.g., aggressive nasal suctioning) and supplemental oxygen as needed.
- The role of chest X-rays in asthma exacerbation is limited unless there is a suspicion for an alternative diagnosis, such as foreign body, concomitant pneumothorax, and/or pneumonia.
- Human rhinovirus and RSV are the most common viral illnesses associated with wheezing early in life and the development of asthma and exacerbations. Up to 85% of asthma exacerbations in children are linked to viral infections, and they contribute to the severity of exacerbations.
- Oxygen saturation should be obtained immediately, and if it remains below 90%, supplemental oxygen should be given. There is no evidence of oxygen suppressing respiratory drives in children with severe asthma.
- Corticosteroid use in acute asthma is associated with reduced inflammation, decreased mucus production, and enhanced beta-agonist activity. Corticosteroid use within one hour of arrival to an emergency department with acute asthma exacerbations reduces the need for hospital admission.
- In severe asthma, consider repeating albuterol nebulized treatment and intravenous magnesium. Intravenous magnesium has been shown to improve pulmonary function in children with acute asthma.
Introduction
Asthma is a chronic disease diagnosed based on patient symptoms that include wheezing, shortness of breath, and cough. The diagnosis is supported by evidence of airway narrowing on spirometer, which is reversible with bronchodilator therapy. Common triggers include infection or allergens.1 An exacerbation is an accentuation of existing inflammatory processes and loss of disease control.2 Among all pediatric age ranges, acute asthma exacerbations are one of the most common reasons for presentation to the emergency department and for hospitalization.3
Epidemiology and Etiology
In the United States alone, 60% of children with diagnosed asthma will have at least one acute exacerbation per year. Up to 20% of those will require a visit to the emergency department annually.3-5 According to the Global Initiative for Asthma guidelines, 300 million individuals have asthma, making it the most common chronic disease of childhood, affecting up to 18% of all pediatric patients and a projected additional 100 million people by 2025.6 Overall, asthma is estimated to account for 1.1% of disability-adjusted life years per 100,000 for all causes.7
A combination of host and environmental factors continues to increase the prevalence and mortality of asthma in children, especially in geographic locations with high air pollution. Host factors, such as atopy, and eczema, and environmental factors (tobacco smoke exposure, air pollution, etc.) have a direct correlation with rising asthma prevalence.6,7 The two major environmental factors involved in the development, persistence, and severity of asthma are airborne pathogens and viral respiratory pathogens.8 There has been evidence that respiratory virus infections in early life frequently are more associated with asthma development. One study noted that an astonishing 50% of infants with respiratory infections caused by respiratory syncytial virus (RSV) during the first 12 months of life developed persistent asthma by school age.9 It remains unclear whether bronchiolitis early in life causes future asthma or simply serves as a marker for susceptibility to pediatric asthma.10
Asthma severity often is divided into four groups: intermittent, persistent mild, persistent moderate, and persistent severe. According to the Expert Panel Report 3 (EPR-3), asthma severity is determined by impairment and risk.11 Impairment is based on effect on day-to-day activities, including daytime symptoms, nighttime awakenings, frequency of short-acting beta-agonist use for relief, interference with normal activities, and spirometry. Risk is based on the number of asthma exacerbations requiring oral systemic steroids per year.
As detailed in Table 1 (available at https://bit.ly/48XIMKD), asthma is considered persistent once the patient is averaging short-acting inhaler use more than two days per week or has minor limitation in normal activities.8 At that point, the patient should be prescribed a daily controller medication, preferentially a low-dose inhaled corticosteroid in addition to a short-acting beta-agonist. (See Table 2, available at https://bit.ly/3vBI3QK).8 According to the Centers for Disease Control and Prevention, 60% of children with current asthma have persistent asthma.
Pathophysiology
Asthma is a complex and dynamic disease revolving around the concept of underlying inflammation, airway hyperresponsiveness, and airway obstruction that causes a variety of symptoms, including wheezing, shortness of breath, coughing, and chest tightness.8 Airway obstruction often results from the constriction of bronchial smooth muscle in response to stimuli, such as allergens and/or irritants. Allergens can trigger an immunoglobulin E (IgE)- or non-IgE-dependent release of histamine, leukotrienes, prostaglandins, and tryptase from mast cells that directly cause airway constriction. Persistent inflammation can produce edema and hypertrophy of smooth muscle in the airway, leading to airway remodeling that is only partially reversible. Inflammation also can contribute to airway hyperresponsiveness or exaggerated bronchoconstrictor response to stimuli. Patients with significant airway remodeling are more prone to this exaggerated response.
In turn, this leads to an increase in emergency department visits for exacerbations unable to be controlled with home rescue therapy. Patients often present to the emergency department hypoxic secondary to ventilation-perfusion mismatch from reflex hypoxic vasoconstriction in addition to mucus plugging and bronchospasm. As seen in Figure 1, there are many potential contributors of inflammation that can trigger acute asthma symptoms, including various interleukins, neutrophils, mast cells, and eosinophils. Treatment is targeted toward reducing inflammation, airway obstruction, hyperresponsiveness, and subsequent long-term remodeling.
Figure 1. Factors Limiting Airflow in Acute and Persistent Asthma |
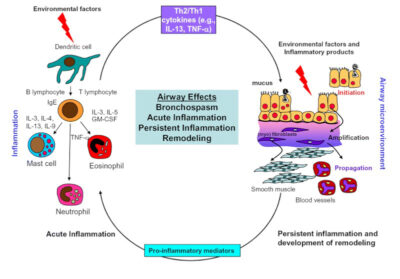 |
Key: GM-CSF, granulocyte-macrophage, colony-stimulating factor; IgE, immunoglobulin E; IL-3, interleukin 3 (and similar); TNF-α, tumor necrosis factor-alpha. Adapted and reprinted from: Holgate ST, Polsa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006;368:780-793, with permission from Elsevier. |
Pediatric patients with and without prior diagnoses of asthma often present to the emergency department with the previously mentioned symptoms of wheezing, shortness of breath, coughing, and chest tightness. The differential for such symptoms is broad, and more challenging in younger patients who are unable to provide a history.
Differential Diagnoses
In addition to asthma, other causes of small airway obstruction presenting with similar symptoms include viral bronchiolitis, bronchopulmonary dysplasia, cystic fibrosis, and congenital heart diseases.
Viral bronchiolitis is the most common lower respiratory tract infection in infants, often presenting with rhinitis, nasal flaring, fever, and tachypnea in addition to wheezing/bronchospasm and cough.13 It is the most common wheezing illness in infancy and must be considered as a primary diagnosis until at least 2 years of age.14 Rhinovirus most often is detected in the spring and fall, compared to RSV in the winter months.14
Unlike asthma, provision of bronchodilators and steroids does not affect the rate or duration of hospitalization in patients with bronchiolitis and who have no history of established asthma, and those medications typically are not indicated.14 Treatment of RSV is primarily supportive therapy (e.g., aggressive nasal suctioning) and supplemental oxygen as needed. Early childhood viral infections can lead to pathophysiologic changes, making children more prone to asthma and subsequent exacerbations. There are several theories as to the mechanism, including changes to the airway microbiome.15
Upper airway obstruction, such as foreign body aspiration, laryngomalacia, vocal cord dysfunction, and vascular rings are other differentials to consider in the acutely dyspneic child, particularly when the patient presents with stridor instead of wheezing. Congenital laryngomalacia is the most common cause of stridor in infants.16 In a pediatric patient with significant tachypnea and accessory muscle use, it often is difficult to distinguish upper airway obstruction from wheezing, resulting in a misdiagnosis of asthma. Dyspnea on exertion, in the absence of other symptoms of asthma or minimal response to albuterol, is most likely the result of a condition other than asthma.17 For these reasons, a thorough history, and cardiopulmonary exam may help avoid improper diagnosis of asthma. When wheezing is present and persistent in the first few months of life without signs of viral illness or any response to therapy, providers must have a high index of suspicion of tracheomalacia and vascular rings.18
Anaphylaxis always must be considered, particularly in patients presenting with impending respiratory failure from suspected acute asphyxic asthma. Patients who are having an anaphylactic reaction but presenting primarily with respiratory symptoms often are misdiagnosed with acute asthma.19 Suspicion for anaphylaxis should increase when patients are slow to respond to bronchodilator therapy after rapid onset of symptoms, particularly wheezing with urticaria and hypotension. These atypical features should lead to a trial of epinephrine for suspected anaphylaxis. Failure to provide epinephrine in a timely manner is the most important factor contributing to mortality in patients presenting with systemic anaphylaxis. The triggers for anaphylaxis often are foods and medications that can be determined at a later time with allergy testing, and such patients are appropriately prescribed self-administering epinephrine devices that have an onset of action within 10 minutes.
Regardless, some overlap remains between anaphylaxis and severe acute asthma, particularly in the setting of allergens that have yet to be identified, leading to underdiagnosis and undertreatment of anaphylaxis in favor of suspected asthma.
Clinical Features
Pediatric asthma exacerbations can have a wide range of presentations, and it is imperative for the provider to recognize key clinical features. The Children’s Hospital of Philadelphia (CHOP) Emergency Department asthma pathway incorporates the Pediatric Asthma Severity Score (PASS) and also uses a rapid assessment to triage patients in descending acuity from emergency severity index (ESI) level 1 to 5. Level 1 patients present with severe wheezing or stridor with severe tachypnea, retractions, grunting respirations, decreased tone, bradypnea, lethargy, absent breath sounds, and agonal respiration with pulse oximetry less than 90%. According to the EPR-3, the patient also may have an absence of wheezing and demonstrate paradoxical thoracoabdominal movement.
ESI Level 2 patients will have decreased aeration with moderate tachypnea, still often with or wheezing and accessory muscle use at rest. Additionally, according to the CHOP pathway, any asthma patient with a history of intubation presenting at triage with suspected asthma exacerbation initially should be at least an ESI level 2 triage.
ESI Level 3 and 4 patients are considered urgent. Level 3 patients have wheezing with only a mild increase in work of breathing with mild retractions and tachypnea. In comparison, ESI Level 4 patients may have wheezing but only minimal work of breathing and initial pulse oximetry higher than 95%. ESI Level 5 patients are considered nonurgent. To view the pathway, visit https://bit.ly/47qMQ50.
Two or more hospitalizations or three or more emergency department visits for asthma in the past year are associated with an increase in the asthma-related mortality risk.8 Other important historical features include onset of symptoms, severity compared to prior exacerbations, intubations, intensive care admissions, and/or complicating cardiac or pulmonary illnesses. Social determinants of health that are associated with an increased risk of mortality in asthma patients include low socioeconomic status or inner-city residence and major psychosocial problems, according to the EPR-3.
A thorough examination is crucial and includes close detail to vital signs and pulse oximetry, hydration status, alertness level, presence of cyanosis, and observation for immediate complications, such as pneumothorax and upper airway obstruction.20 It is essential to consider additional diagnoses, such as a foreign body, before proceeding to additional asthma-targeted therapy, particularly in pediatric patients presenting acutely ill who do not respond to initial therapy.
There typically is a delay in presentation for children who aspirate foreign bodies, which can make the diagnosis even more elusive. Approximately 50% to 70% of patients present within the first 24 hours of the aspiration event. The provider must have a high degree of clinical suspicion and ask caregivers about any recent choking events. On physical exam, providers should assess for decreased breath sounds on one side, usually right-sided given the angle of the bronchus, and lack of improvement with asthma treatment. It also is important to consider that, although only 20% of foreign bodies are radiopaque, abnormal findings are present in 40% to 80% of cases. If there is a suspicion for foreign body, perform a two-view X-ray. (See Figure 2.) Either inspiratory/expiratory films or decubitus vies which will demonstrate air trapping on the affected side.
Figure 2. Chest X-Ray with Bronchial Foreign Body |
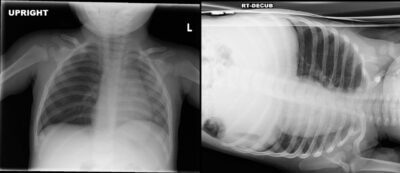 |
Images courtesy of Aaron Leetch. Pediatric Emergency Medicine Reports, January 2016. |
Clinical Scoring Systems
There are many different scoring systems used for asthma exacerbations, including PASS, Respiratory Score (RS), and Pediatric Asthma Score (PAS). The PASS assesses three categories of clinical findings, including wheezing, work of breathing, and prolongation of expiration. PASS uses a score of 0 for mild, 1 for moderate, and 2 for severe findings in each category.
For example, mild wheezing would be given a score of 0, moderate wheezing would be given a score of 1, and severe wheezing — or absent wheezing because of poor air movement — would be given a score of 2. A combined PASS score of < 8 indicates a low-risk patient, a PASS score of 8-11 indicates a medium-risk patient, and a PASS score > 11 represent a high-risk patient. The scoring system has been validated for use in pediatric patients from 1 to 18 years of age, with scores assigned on initial assessment and after emergency department treatment.
Additionally, pulse oximetry for assessment of hypoxemia also is an important feature in the disposition and severity of patients with asthma. Supplemental oxygen should be provided to maintain oxygen above 90%.
The benefits of the PASS system include its ease of use, minimal cost, and reliability among providers from nurses to respiratory therapists to physicians. Additionally, the scores before and after treatment were consistent with other measures of asthma severity, such as peak expiratory flow rate (PEFR), and were responsive to patient status during emergency department treatment. Unlike several other scoring systems, the PASS has been tested in a large patient population with a broad age range, exacerbation severity, and without exclusions for severe exacerbation or inability to perform PEFR.21
The biggest limitation of the PASS is that it is based on only three categories, which may cause hesitation in some clinicians. Alternatively, the RS provides a more detailed scoring system with four categories: respiratory rate, retractions, dyspnea, and auscultation. The RS is scored from 0 to 3 points, with a maximum total of 12 points. Both respiratory rate and dyspnea are subdivided into age groups, while retractions and auscultation are applied similarly to all patients. Based on the limited data used to study the RS system, the PASS has been shown to decrease emergency department length of stay. However, there is no shown decrease in inpatient or intensive care admissions or inpatient length of stay.22
The acute asthma pathway developed at Seattle Children’s Hospital involves taking an RS at triage and every hour afterward. For example, an RS of less than 6 at triage involves steroid dose and albuterol given via metered dose inhaler (MDI). Meanwhile, a score of 6 or more results in the patient receiving a steroid dose and albuterol continuous nebulization therapy, with ipratropium for patients older than 2 years of age. Similarly, there are additional decision-making pathways following the RS score at hour 2, divided into an RS of less than 5, from 5 to 8, and more than 8.23
Compared to the PASS, RS provides more specific management recommendations, since it was originally intended to guide inpatient management of asthma exacerbations. Similarly, the PAS also was originally developed to guide inpatient management of asthma exacerbations, but it has been extended to the emergency department. The PAS has five categories: respiratory rate, oxygen requirement, auscultation, retractions, and dyspnea, with a maximum score of 15. Scores of 5-7 are considered mild exacerbations, scores of 8-11 are considered moderate, and scores of 12-15 are considered severe. Although primary data for the PAS was based on a small sample size and retrospective studies, the use of PAS, as well as asthma education, was found to decrease costs, increase the likelihood of oral steroid dose vs. intravenous steroids, improve follow-up, and also increase rates of a controller medication being prescribed upon discharge.24
Asthma scoring systems have been found to be useful in guiding management and disposition but are not meant to replace clinical judgement. For example, it is possible for a patient with hypoxemia to have a minimal increase in work of breathing and distress, which may provide a low RS and inappropriate reassurance. In this situation, clinical judgment must have a significant role. For this reason, several institutions, such as CHOP, include a section for history and assessment into their emergency department pathway for asthma exacerbations. This includes a brief history and examination, as previously discussed. CHOP’s chart used to determine a patient’s level of asthma exacerbation is available at https://bit.ly/3vz9oD3.
Diagnostic Studies
The role of chest X-rays in asthma exacerbation is limited unless there is a suspicion for an alternative diagnosis, such as foreign body, concomitant pneumothorax, and/or pneumonia. In patients with respiratory distress consistent with asthma, the likelihood of pneumonia found on chest X-ray is very low.25 A retrospective study analyzing more than 1,500 asthma visits over a two-year period found that chest X-rays were obtained by the physician in nearly 400 of those visits.25 The most associated pretest indicators for obtaining a chest X-ray in this patient group was fever greater than 38.3°C, symptoms lasting longer than two days, patient age less than 5 years, respiratory rate greater than 40 breaths per minute, or pulmonary rales. Of the chest X-rays ordered, 10% had an infiltrate and 5% had atelectasis. In this study, only patients aged younger than 5 years and patients presenting with pulmonary rales were found to be associated with abnormal radiographs. No significant difference was found in the X-rays of patients treated as outpatients and those requiring hospitalization.25
A more recent retrospective study on children with acute asthma exacerbations who received a chest X-ray in a pediatric emergency department demonstrated that, among patients with various clinical or historical features, only those who had received previous antibiotic administration in the past seven days had both a significant relationship with both pneumonia found on chest X-ray and antibiotic administration in the emergency department.26 The data, although retrospective in nature, suggest that chest X-ray utility is limited in terms of disposition and management of suspected asthma exacerbations presenting to the emergency department.
As the role of chest X-ray in acute asthma exacerbation diminishes, research in the use of ultrasound continues to evolve. Point-of-care ultrasound also lends itself to the identification of alternative diagnoses, such as myocarditis and/or evidence of congenital heart disease, as well as pulmonary edema. More specifically, point-of-care ultrasound can accurately detect pneumothorax, subpleural or consolidative pneumonia, and focal atelectasis, offering a fast, cost- and radiation-effective tool that helps guide management.27
In 2017, a cross-sectional study by Dankoff et al attempted to categorize lung ultrasound findings in children with asthma presenting to the emergency department with respiratory distress. The study sonographer, who was a novice to lung ultrasound, attended a two-day point-of-care ultrasound course to learn a six-zone linear transducer protocol examining bilateral anterior, mid-axillary, and posterior chest zones. Children aged 2-17 years who were determined to have at least a moderate asthma exacerbation by a nurse-driven, physician-blinded protocol were evaluated after receiving steroid and beta-agonist treatment. A positive ultrasound was defined as multiple B-lines, consolidation, or pleural abnormalities. A negative lung ultrasound was defined as the presence of A-lines without other findings.27 (See Figure 3.)
Figure 3. Examples of Positive Lung Findings Described |
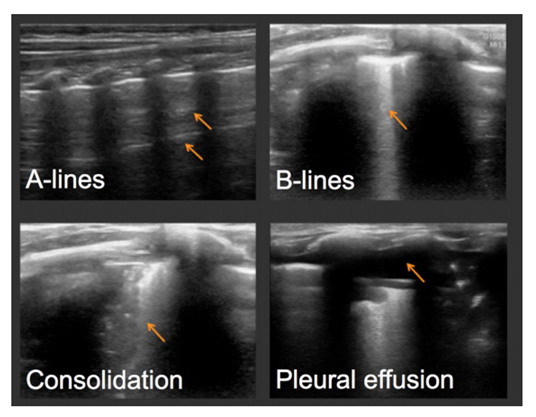 |
Reprinted with permission from Dankoff S, Li P, Shapiro AJ, et al. Point-of-care lung ultrasound of children with acute asthma exacerbations in the pediatric ED. Am J Emerg Med 2017;35:615-622. |
In all, 90% of all study patients were diagnosed with a pure asthma exacerbation by the physician, 41% of whom had positive ultrasounds as determined by the sonographer. On the contrary, 10% were diagnosed with both asthma and pneumonia, and 85% of those patients had positive lung ultrasounds. Overall, a positive lung ultrasound correlates to prolonged length of stay in the ED, admission rate, and additional therapies, such as oxygen and magnesium.27 In contrast to chest X-ray, early evidence shows there may be a role for point-of-care ultrasound in patient disposition and earlier goal directed treatment.
Like ultrasound and chest X-rays, there may be a role for viral panel testing in pediatric patients presenting to the emergency department for suspected acute asthma exacerbations, particularly those patients who are likely to be admitted. Human rhinovirus (RV) and RSV are the most common viral illnesses associated with wheezing early in life and the development of asthma and exacerbations. Up to 85% of asthma exacerbations in children are linked to viral infections, and they contribute to the severity of exacerbations.15,28
However, there remains no strong objective clinical data supporting viral polymerase chain reaction testing in non-hospitalized pediatric asthma exacerbations.29 Chang et al examined children 2 to 15 years of age presenting to the emergency department for acute asthma exacerbations who were not hospitalized. The authors found no clinically significant association with quality-of-life score on presentation to the emergency department, day 7, or day 14 of illness.29 Viruses assessed in this study included influenza, RSV, RV, adenovirus, parainfluenza, coronavirus subtypes, as well as the bacteria Chlamydia and Mycoplasma.
Respiratory viruses are present in most patients hospitalized for acute asthma and life-threatening asthma and may contribute to mortality in these patients.30
According to analysis of a prospective cohort of nearly 1,000 children presenting to the emergency department with moderate to severe asthma exacerbations, respiratory pathogens were not associated with higher severity on presentation to the emergency department, but they were associated with an increased treatment failure risk, defined as hospital admission, emergency department stay for more than eight hours, or relapse presentation. Parainfluenza was associated with the highest treatment failure risk at nearly 35%.31 Viral testing in hospitalized pediatric patients also assists in disposition/inpatient management and cohorting through respiratory precautions, contributing to the safety of surrounding patients and family members. For this reason, hospitalized pediatric patients with acute asthma exacerbations likely would benefit from viral testing.
Management Approach
Implementing a clinical pathway for pediatric emergency department asthma management leads to improved adherence to evidence-based practice and fewer hospital admissions.32
Studies from Canada, South America, and the United Kingdom have shown that pediatric asthma exacerbation pathways not only decrease hospitalization rates, but they also decrease prescribing errors and return visits. However, in the United States, pediatric asthma management in the emergency department is variable, with pathway implementation often limited by a lack of follow-up options and a fee-for-service system. It should be acknowledged that there are many different asthma pathways available, and there is no evidence to support the superiority of one pathway over another.
The benefits of implementing an asthma pathway come from the standardization and expedition of care, including timely administration of steroids and bronchodilators, which are known to decrease admission rates. Every 30-minute delay in emergency department steroid administration past one hour is associated with a 20% increase in the odds of admission for pediatric asthma exacerbation. In this review, the CHOP Pediatric Emergency Department Asthma pathway is emphasized. CHOP has a robust clinical pathways program led by multidisciplinary care teams. CHOP has more than 100 pathways, with four such pathways on different phases of asthma care alone. Furthermore, the CHOP pathway incorporates the PASS system.
When a patient arrives in the triage area of the emergency department with a suspected acute asthma exacerbation, the initial step should focus on rapid assessment and assigning a nursing ESI level of 1 through 5 in order of descending acuity level, followed by a brief assessment of pertinent history and examination. One of the most important components of the initial screening is evaluating and reversing hypoxemia. One set of criteria cannot define severity accurately, given the wide spectrum of disease. Pulmonary function tests are objective, but they require both appropriate effort by the patient and must be interpreted with consideration of baseline values, if available, or predicted value based on height. Additionally, they usually are not possible in children younger than 5 or 6 years of age. This highlights pulse oximetry as an invaluable component of the initial assessment because it provides objective and real-time assessment of overall asthma severity. Oxygen saturation should be obtained immediately, and if it remains below 90%, supplemental oxygen should be given. 33 There is no evidence of oxygen suppressing respiratory drives in children with severe asthma.34
Several options are available for noninvasive oxygen therapy, including nasal cannula, high-flow nasal cannula (HFNC), simple masks, nonrebreather masks, and continuous and bilevel positive airway pressure (CPAP and BIPAP). (See Table 3.)
Table 3. An Overview of Oxygen Delivery Methods | |||||
Oxygen Delivery Method | Oxygen Flow Rate | FiO2 | Design | Advantages | Limitations |
Nasal cannula |
| 40% | Nasal prongs |
|
|
High-flow nasal cannula |
| 100% | Nasal prongs, heated and humidified |
|
|
Simple mask |
| 60% | Face mask |
|
|
Nonrebreather mask |
| 100% | Mask with one-way valve, reservoir bag |
|
|
Continuous positive airway pressure |
| 100% | Face or nasal mask |
|
|
Bilevel positive airway pressure |
| 100% | Face or nasal mask |
|
|
Simple masks can provide up to 10 L of flow per minute and a fraction of inspired oxygen (FiO2) up to 60%. Nonrebreather masks deliver up to 15 L of flow per minute and an FiO2 close to 100%.35 Of note, at lower flow rates, a nonrebreather mask could lead to an ineffective washout of exhaled carbon dioxide and the potential to rebreathe exhaled gases. A nasal cannula can provide an FiO2 up to 40% with 6 L of flow per minute.
Although the definition of HFNC varies, flow rates of more than 6 L/ minute up to 30 L/minute generally are considered high flow in children, whereas flow rates of 2 L/minute adjusted up to 2 L/kg/minute are considered high flow in infants, with FiO2 approaching 100%. In addition to increased flow rates and potential FiO2 in HFNC, the mixture of air is heated and humidified, which decreases mouth dryness and resistance in the nasal mucosa, improving patient tolerance and oxygen flow rates to distal airways.36 Furthermore, HFNC can create a positive end expiratory pressure (PEEP) up to 6 cm H2O during expiration, similar to CPAP. Several potential drawbacks of HFNC exist. In theory, lack of precise measurement of pressures generated in the airways could contribute to pneumothorax risk.
Additionally, although nebulized albuterol can be given in line with an HFNC circuit, drug delivery decreases with an increase in flow rate, an issue that can be avoided altogether with CPAP and BIPAP.34 CPAP and BIPAP are administered using conventional ventilators and create positive pressure through a face mask or nasal mask that distends the distal airways to improve lung oxygenation and volumes while decreasing work of breathing.33 CPAP delivers one constant pressure throughout the respiratory cycle, typically starting at 4 cm to 6 cm H2O and titrates based on work of breathing. BIPAP requires two pressure settings, with inspiratory positive airway pressure (IPAP) typically starting at 10 cm to 12 cm H2O and expiratory positive airway pressure (EPAP) typically starting at 4 cm to 6 cm H2O.
Heliox is an option for severe exacerbations. With narrowing of the airways in acute asthma, the velocity of gas in the airway increases and leads to turbulent flow, leading to increased airway resistance and, thus, decreased deposition of inhaled oxygen and medication to distal alveoli. Heliox is oxygen mixed with helium, which is a gas that is much less dense than air, leading to increased distal laminar flow. A recent meta-analysis has shown an improvement in asthma severity scores following two hours of heliox use in pediatric patients with severe exacerbations, particularly with a mixture of 70% helium and 30% oxygen.3
Following the initial rapid assessment and assignment of PASS to determine the level of severity, treatment should be initiated accordingly. If the patient is determined to be an ESI level 4, with mild symptoms and PASS of 0 or 1, consider oral dexamethasone and two to four puffs of an albuterol MDI. Clinicians can anticipate discharge if there is improvement in the patient’s symptomatology. When working with an ESI level-3 patient (with a PASS score of less than 3), clinicians should consider dexamethasone provisioning along with albuterol MDI every 20 minutes for three total doses. Reassess after an hour without any treatment for symptom recurrence. If symptoms one hour post treatment are mild, the provider may consider discharging the patient.
Patients with severe exacerbations are likely to be taken to the resuscitation bay from triage, then given a steroid dose and albuterol ipratropium combination nebulizer treatment along with supplemental oxygen for hypoxemia. Beta-agonists, such as albuterol, cause bronchodilation through beta-2 receptor activated bronchial smooth muscle relaxation.36 Ipratropium is an atropine derivative that aids in bronchodilation by inhibiting cholinergic mediated bronchospasm and is an important agent in treating moderate to severe asthma in the emergency department.37,38 Ipratropium reduces the rate of hospitalization when given with second and third albuterol doses, presuming a steroid dose was given on arrival.39
Corticosteroid use in acute asthma is associated with reduced inflammation, decreased mucus production, and enhanced beta-agonist activity.40 Corticosteroid use within one hour of arrival to an emergency department with acute asthma exacerbations reduces the need for hospital admission, with a number needed to treat of 8.41 More specifically, as it relates to asthma pathways, triage nurse initiation of oral corticosteroids in children with moderate to severe asthma exacerbations, prior to physician assessment, has been associated with earlier clinical improvement, discharge, and reduced admission rates.42
According to the CHOP pathway, steroid options are oral or intravenous prednisone (2 mg/kg up to a maximum of 60 mg) or a dexamethasone tablet in a mild to moderate flare-up, stratified by weight, often crushed in juice or syrup. More specifically, give a 4-mg tablet for children weighing 5 kg to 8 kg, a 6-mg tablet for children weighing 8 kg to 12 kg, and an 8-mg tablet for children weighing more than 12 kg. A single dose of intramuscular dexamethasone 0.6 mg/kg showed no clinically significant difference in outcomes compared to a five-day course of oral prednisone for treatment of moderate acute asthma exacerbations discharged from the emergency department.43 Subsequent studies showed no significant difference between intramuscular or oral dexamethasone.41 In addition to shorter course of therapy, decreased costs, and implied increase in compliance, patients receiving dexamethasone compared to prednisone had significantly decreased instances of vomiting in the emergency department or at home following discharge.44
In severe asthma exacerbations not responsive to an initial bronchodilator, next-tier therapy is indicated. This includes consideration of continuous nebulized therapy, terbutaline, epinephrine, and intravenous magnesium. In severe asthma, defined as a PASS score greater than 3, consider repeating albuterol nebulized treatment and intravenous magnesium. Intravenous magnesium has been shown to improve pulmonary function in children with acute asthma when given at 50 mg/kg up to a maximum of 1,500 mg over 60 minutes.31 The CHOP pathway extends the maximum dose to 2,000 mg. Intravenous magnesium also has been shown to reduce the odds of hospital admissions for acute pediatric asthma with no significant side effects, according to recent reviews.32,45 The mechanism of action of magnesium is theorized to be blockage of N-methyl-D-aspartate receptor-gated calcium channels, resulting in muscle relaxation and associated bronchodilation.46-48
Concerns remain about magnesium-induced muscle weakness and pervasive vasodilation leading to respiratory failure and hypotension, but this is not supported with evidence. Although earlier studies showed minimal or no adverse effects, fear of potential side effects remains widespread.49 To encourage the use of magnesium, the CHOP pathway for administration of magnesium recommends a 20 mL/kg bolus of normal saline given in parallel, with vital signs taken every 15 minutes and emergency department observation for one hour prior to inpatient floor transfer. The bolus of fluids typically is beneficial in patients with refractory severe disease to account for poor oral hydration and fluid losses. The results of a randomized controlled trial with 100 patients in India showed early intravenous magnesium sulfate was superior to terbutaline, determined by a modified asthma clinical severity score.50
In addition, the CHOP asthma pathway suggests early terbutaline for severe asthma exacerbations with a concern for impending respiratory failure. Subcutaneous initial treatment before intravenous line placement, and intermittent boluses after placement, is preferred. For patients aged 12 years and older, start with a subcutaneous dose of 0.25 mg delivered into the lateral deltoid area. If significant improvement does not occur within 15 to 30 minutes, a second dose of 0.25 mg can be administered. The maximum dose is 0.5 mg within four hours. For intravenous boluses, the dosage is 10 mcg/kg (maximum 250 mcg) every 15-30 minutes × 3.
Intravenous infusions are not recommended, and it is advised to use repeat boluses as needed instead. If necessary, start at a rate of 0.4 mcg/kg/minute with a loading dose of 10 mcg/kg (max 400 mcg), then titrate to 3 mcg/kg/minute.
An intravenous beta-2-agonist should be considered in patients not improving on continuous albuterol. In the United States, terbutaline is the intravenous beta-2-agonist of choice.51 Of note, when terbutaline is given subcutaneously, it loses beta selectivity and has no perceived advantage over epinephrine.52 Previous studies showed that when given in a dose of 0.01 mg/kg with a maximum dose of 0.25 mg, both epinephrine and terbutaline resulted in bronchodilation within five minutes that was sustained for four hours. No clinically significant side effects were noted.51 Potential side effects of beta-agonists include tachycardia, diastolic hypotension, arrhythmias, QT prolongation, and hypokalemia.35,54
For patients with severe asthma failing to improve or deteriorating despite optimization of conventional therapy, noninvasive positive pressure ventilation has been shown to improve clinical exam and gas exchange, often in patients as young as 1 year of age.55
The combination of air trapping, bronchospasm, and airway obstruction leads to severe lung hyperinflation and increased positive intrapleural pressure. This positive pressure decreases passive air movement and leads to tachypnea, shortened inspiration, and worsening hyperinflation.56 Noninvasive ventilation methods, including HFNC, CPAP, and BIPAP, oppose intrinsic PEEP and recruit previously collapsed alveoli to increase total lung capacity toward baseline.57 BIPAP also may increase delivery of nebulized albuterol to areas of poor ventilation.58 With an increased inspiratory positive airway pressure, a decreased expiratory positive airway pressure, and a prolonged inspiration to expiration ratio, there are fewer endotracheal intubations and pediatric intensive care unit admissions and overall improvements in PASS scores.57
Although large randomized studies are lacking, many small studies and case reports suggest that a combination of ketamine (244 patients aged 5 months to 70 years who received ketamine for bronchospasm) and BIPAP may be beneficial in severe asthma exacerbations and may help avoid mechanical ventilation.59,60 Ketamine given at a loading dose of 1 mg/kg followed by a continuous infusion for one to two hours has been associated with an improvement in asthma scores, oxygen saturation, and mean peak airway pressures.60 Ketamine is a phencyclidine derivative that blocks N-methyl-D-aspartate receptor-induced bronchospasm through smooth muscle relaxation and depression of nitric oxide levels and inflammatory markers.61,62 The known dissociative effects of ketamine without suppression of the respiratory drive increase chest wall compliance and overall synchrony with noninvasive or invasive ventilation.60,63 Ketamine also has favorable pharmacologic properties, including onset of action within 60 seconds and a single bolus duration of action up to 15 minutes.64 The most common side effects of ketamine often are treatable or preventable, including increased airway secretions and hallucinations, addressed with atropine and benzodiazepines, respectively.60 Although infrequent, laryngospasm, and apnea can occur with higher doses or rapid administration.64,65 If laryngospasm occurs, proceed with a chin lift, jaw thrust technique followed by CPAP and assessment of air entry and bag movement. If the patient remains in complete laryngospasm, administer propofol to deepen anesthesia for subsequent endotracheal intubation.
The decision to progress from pharmacologic and noninvasive therapy to intubation and mechanical ventilation is based on clinical judgment.66,67 Studies support a wide variability in practice, but the decision should not be delayed once deemed necessary. Signs of hypercarbic and hypoxic respiratory failure with altered mental status and metabolic acidosis from anaerobic metabolism despite optimization are signs that intubation and mechanical ventilation are necessary. Permissive hypercapnia, increase in expiratory time, and ventilator synchrony are the mainstay of mechanical ventilation. Neither a pressure- nor volume-targeted mode of ventilation has been proven to be superior, but the goal is to limit barotrauma and hyperinflation while providing adequate gas exchange.3,33,68 The ideal method for intubation in these patients is to have the most seasoned airway expert available perform a rapid sequence intubation, given the propensity of patients with severe asthma to rapidly desaturate and the high risk for cardiopulmonary collapse.
Hyperinflation occurs because of breath stacking, or unintended high tidal volumes from incomplete exhalation between inspiratory cycles. Hyperinflation is best determined by end inspiratory plateau pressure and positive end expiratory pressure. The American College of Chest Physicians recommends maintaining end inspiratory plateau pressure of less than 35 cm H2O.67,69 Following intubation, hypotension commonly occurs secondary to hyperinflation but also is exacerbated by a combination of sedation and hypovolemia.33 Controlled hypoventilation, also known as permissive hypercapnia, is essential in the immediate post-intubation period to reduce hyperinflation. It often is combined with increased expiratory time and continuous bronchodilator therapy to decrease expiratory flow resistance.70,71
Discharge
Comprehensive discharge planning is essential for pediatric patients treated for acute asthma in the emergency department. The CHOP pathway emphasizes family viewing of an asthma education video, consideration of starting a controller medication, MDI instruction, follow-up appointments with a primary care physician, and strict return precautions. MDIs with holding chambers produce outcomes that are at least equivalent to nebulizer therapy for inhaled corticosteroids and beta-agonists in acute asthma. Proper MDI use may prevent emergency department visits for some mild to moderate cases of acute asthma, which further highlights the importance of proper MDI use.72 Scheduling an appointment with a primary care physician after an emergency department visit has been demonstrated to increase the likelihood that urban children with asthma would follow up with a provider.73 Follow-up with the patient’s primary care physician is recommended within three to five days and provides an opportunity for reassessment, discussion of an asthma plan, and possible controller medication therapy. The development of an asthma action care plan is important because it also may reduce future acute exacerbations.3
The outpatient follow-up rates after emergency department visits for acute asthma remain low.74 One solution would be to prescribe or provide an inhaled corticosteroid controller medication in the emergency department at discharge. It has been suggested that this approach is more cost-effective than relying on outpatient follow-up, reduces bounce-back visits to the emergency department, and results in higher rates of controller medication initiation.74,75 A recent study found that there was an increased likelihood of follow-up with the primary care physician within four weeks of the emergency department visit if the parents believed the child had “very severe” asthma and were using a daily controller medication.76 Furthermore, more than 80% of pediatricians support initiation of corticosteroid controller medications in the emergency department.77 Another proactive measure that can be implemented in the emergency department is influenza vaccination programs, since vaccination among children with asthma remains low despite increased complication rates among those with asthma.78,79 Children with asthma represent more than one-third of pediatric patients admitted for influenza, despite an asthma prevalence rate of 10%.78
Conclusion
Acute asthma exacerbations represent a large proportion of emergency department visits among children — with significant mortality and cost to the healthcare system. The implementation of standardized pathways incorporating validated asthma severity scoring systems continues to expand, with an emphasis on rapid triage and early goal-directed therapy involving steroids and bronchodilator therapy. The role of chest X-ray continues to diminish while the use of point-of-care ultrasound increases.
Management of the acutely dyspneic child with suspected asthma should include appropriate provision of oxygen and adjunctive pharmacologic therapy, including the use of albuterol, ipratropium, corticosteroids, and consideration of magnesium, terbutaline, heliox, and ketamine. However, progression to intubation should not be delayed in patients with worsening hypercarbic and hypoxic respiratory failure and with altered mental status. When discharging patients, provide appropriate follow-up and instructions and consider prescribing controller medications in patients with persistent features.
References
- Van Aalderen WM. Childhood asthma: Diagnosis and treatment. Scientifica 2012;2012:674204.
- Castillo JR, Peters SP, Busse WW. Asthma exacerbations: Pathogenesis, prevention, and treatment. J Allergy Clin Immunol Pract 2017;5:918-927.
- Jones BP, Fleming GM, Otillio JK, et al. Pediatric acute asthma exacerbations: Evaluation and management from emergency department to intensive care unit. J Asthma 2016;53:607-617.
- Dondi A, Calamelli E, Piccinno V, et al. Acute asthma in the pediatric emergency department: Infections are the main triggers of exacerbations. Biomed Res Int 2017;2017:9687061.
- Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3 2012;3:1-58.
- Masoli M, Fabian D, Holt S, Beasley R; Global Initiative for Asthma Program. The global burden of asthma: Executive summary of the GINA Dissemination Committee Report. Allergy 2004;59:469-478.
- Ferrante G, La Grutta S. The burden of pediatric asthma. Front Pediatr 2018;6:186.
- National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma — Summary Report 2007. J Allergy Clin Immunol 2007;120(5 Suppl):S94-S138.
- Bacharier LB, Cohen R, Schweiger T, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2012;130:91-100.
- Beigelman A, Bacharier LB. The role of early life viral bronchiolitis in the inception of asthma. Curr Opin Allergy Clin Immunol 2013;13:211-216.
- Pollart SM, Edward KS. Overview of changes to asthma guidelines: Diagnosis and screening. Am Fam Physician 2009;1:761-767.
- Centers for Disease Control and Prevention. Asthma severity among children with current asthma. Reviewed Feb. 17, 2015. https://www.cdc.gov/asthma/asthma_stats/severity_child.htm
- American Academy of Pediatrics Subcommittee on Diagnosis and Management of Bronchiolitis. Diagnosis and management of bronchiolitis. Pediatrics 2006;118:1774-1793.
- Gern JE. Viral respiratory infection and the link to asthma. Pediatr Infect Dis J 2008;27(10 Suppl):S97-S103.
- Kim CK, Callaway Z, Gern JE. Viral infections and associated factors that promote acute exacerbations of asthma. Allergy Asthma Immunol Res 2018;10:12-17.
- Tamura K, Shirai T, Matsubara A. Laryngomalacia presenting as severe uncontrolled asthma. Respirol Case Rep 2018;6:e00316.
- Weinberger M, Abu-Hasan M. Pseudo-asthma: When cough, wheezing, and dyspnea are not asthma. Pediatrics 2007;120:855-864.
- Tenero L, Piazza M, Piacentini G. Recurrent wheezing in children. Transl Pediatr 2016;5:31-36.
- Rainbow J, Browne G. Fatal asthma or anaphylaxis? Emerg Med J 2002;19:415-417.
- Zorc J, Scarfone R, Reardon A, et al. Asthma clinical pathway — emergency. Revised May 2023. https://www.chop.edu/clinical-pathway/asthma-emergent-care-clinical-pathway
- Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med 2004;11:10-18.
- Rutman L, Migita R, Spencer S, et al. Standardized asthma admission criteria reduce length of stay in a pediatric emergency department. Acad Emerg Med 2016;23:289-296.
- Ken L, Migita R, Burns B; Seattle Children’s Hospital. Asthma pathway. Revised April 2023. https://www.seattlechildrens.org/pdf/asthma-pathway.pdf
- Kelly CS, Andersen CL, Pestian JP, et al. Improved outcomes for hospitalized asthmatic children using a clinical pathway. Ann Allergy Asthma Immunol 2000;84:509-516.
- Rushton AR. The role of the chest radiograph in the management of childhood asthma. Clin Pediatr (Phila) 1982;21:325-328.
- Allie EH, Dingle HE, Johnson WN, et al. ED chest radiography for children with asthma exacerbation is infrequently associated with change of management. Am J Emerg Med 2018;36:769-773.
- Dankoff S, Li P, Shapiro AJ, et al. Point-of-care lung ultrasound of children with acute asthma exacerbations in the pediatric ED. Am J Emerg Med 2017;35:615-622.
- Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9-11-year-old children. BMJ 1995;310:1225-1229.
- Chang AB, Clark R, Acworth JP, et al. The impact of viral respiratory infection on the severity and recovery from an asthma exacerbation. Pediatr Infect Dis J 2009;28:290-294.
- Özdemir A, Doğruel D. Efficacy of magnesium sulfate treatment In children with acute asthma. Med Princ Pract 2020;29:292-298.
- Merckx J, Ducharme FM, Martineau C, et al. Respiratory viruses and treatment failure in children with asthma exacerbation. Pediatrics 2018;142:e20174105.
- Colleti Jr J, de Carvalho WB. Magnesium sulfate for acute asthma in children. Pediatr Crit Care Med 2016;17:477-478.
- Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: Severe asthma. Crit Care 2002;6:30-44.
- Schiff M. Control of breathing in asthma. Clin Chest Med 1980;1:85-89.
- Stephanopoulos DE, Monge R, Schell KH, et al. Continuous intravenous terbutaline for pediatric status asthmaticus. Crit Care Med 1998;26:1744-1748.
- Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med 1998;158:S146-S153.
- Gross NJ. Ipratropium bromide. N Engl J Med 1988;319:486-494.
- Plotnick LH, Ducharme FM. Should inhaled anticholinergics be added to beta2 agonists for treating acute childhood and adolescent asthma? A systematic review. BMJ 1998;317:971-977.
- Zaritsky A, Qureshi F. Ipratropium does indeed reduce admissions to hospital with severe asthma. BMJ 1999;318:738.
- Keeney GE, Gray MP, Morrison AK, et al. Dexamethasone for acute asthma exacerbations in children: A meta-analysis. Pediatrics 2014;133:493-499.
- Rowe BH, Spooner C, Ducharme F, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev 2001;2001:CD002178.
- Zemek R, Plint A, Osmond MH, et al. Triage nurse initiation of corticosteroids in pediatric asthma is associated with improved emergency department efficiency. Pediatrics 2012;129:671-680.
- Gordon S, Tompkins T, Dayan PS. Randomized trial of single-dose intramuscular dexamethasone compared with prednisolone for children with acute asthma. Pediatr Emerg Care 2007;23:521-527.
- Kim MK, Yen K, Redman RL, et al. Vomiting of liquid corticosteroids in children with asthma. Pediatr Emerg Care 2006;22:397-401.
- Irazuzta JE, Chiriboga N. Magnesium sulfate infusion for acute asthma in the emergency department. Jornal de Pediatria (Versão em Português) 2017;93:19-25.
- del Castillo J, Engbaek L. The nature of the neuromuscular block produced by magnesium. J Physiol 1954;124:370-384.
- Gourgoulianis KI, Chatziparasidis G, Chatziefthimiou A, Molyvdas PA. Magnesium as a relaxing factor of airway smooth muscles. J Aerosol Med 2004;14:301-307.
- Yoshioka H, Hirota K, Sato T, et al. Spasmolytic effect of magnesium sulfate on serotonin-induced pulmonary hypertension and bronchoconstriction in dogs. Acta Anaesthesiol Scand 2001;45:435-440.
- Devi PR, Kumar L, Singhi SC. Intravenous magnesium sulfate in acute severe asthma not responding to conventional therapy. Indian Pediatr 1997;34:389-397.
- Singhi S, Grover S, Bansal A, Chopra K. Randomised comparison of intravenous magnesium sulphate, terbutaline and aminophylline for children with acute severe asthma. Acta Paediatr 2014;103:1301-1306.
- Nievas IF, Anand KJ. Severe acute asthma exacerbation in children: A stepwise approach for escalating therapy in a pediatric intensive care unit. J Pediatr Pharmacol Ther 2013;18:88-104.
- Amory DW, Burnham SC, Cheney Jr FW. Comparison of the cardiopulmonary effects of subcutaneously administered epinephrine and terbutaline in patients with reversible airway obstruction. Chest 1975;67:279-286.
- Sly R, Badiei B, Faciane J. Comparison of subcutaneous terbutaline with epinephrine in the treatment of asthma in children. J Allergy Clin Immunol 1977;59:128-135.
- Haalboom JR, Deenstra M, Struyvenberg A. Hypokalaemia induced by inhalation of fenoterol. Lancet 1985;1:1125-1127.
- Thill PJ, McGuire JK, Baden HP, et al. Noninvasive positive-pressure ventilation in children with lower airway obstruction. Pediatr Crit Care Med 2004;5:337-342.
- Martin JG, Shore SA, Engel LA. Mechanical load and inspiratory muscle action during induced asthma. Am Rev Respir Dis 1983;128:455-460.
- Abramo T, Williams A, Mushtaq S, et al. Paediatric emergency department BiPAP continuous quality improvement programme with patient analysis: 2005-2013. BMJ Open 2017;7:e011845.
- Smith TC, Marini JJ. Impact of PEEP on lung mechanics and work of breathing in severe airflow obstruction. J Appl Physiol (1985) 1988;65:1488-1499.
- Kiureghian E, Kowalski J. Intravenous ketamine to facilitate noninvasive ventilation in a patient with a severe asthma exacerbation. Am J Emerg Med 2015;33:1720.e1-1720.e2
- Goyal S, Agrawal A. Ketamine in status asthmaticus: A review. Indian J Crit Care Med 2013;17:154-161.
- Sato T, Hirota K, Matsuki A, et al. The role of the N-methyl-D-aspartic acid receptor in the relaxant effect of ketamine on tracheal smooth muscle. Anesth Analg 1998;87:1383-1388.
- Zhu M-M, Qian Y-N, Zhu W, et al. [Protective effects of ketamine on allergen-induced airway inflammatory injure and high airway reactivity in asthma: Experiment with rats.] Zhonghua Yi Xue Za Zhi 2007;87:1308-1313.
- Jat KR, Chawla D. Ketamine for management of acute exacerbations of asthma in children. Cochrane Database Syst Rev 2012;11:CD009293.
- Stevenson C. Ketamine: A review. Update in Anaesthesia 2005;20:25-29.
- Reich DL, Silvay G. Ketamine: An update on the first twenty-five years of clinical experience. Can J Anaesth 1989;36:186-197.
- Bratton SL, Newth CJ, Zuppa AF, et al. Critical care for pediatric asthma: Wide care variability and challenges for study. Pediatr Crit Care Med 2012;13:407-414.
- Slutsky AS. Mechanical ventilation. Chest 1993;104:1833-1859.
- Bohn D, Kissoon N. Acute asthma. Pediatr Crit Care Med 2001;2:151-163.
- Corbridge TC, Hall JB. Status asthmaticus. In: Hall JB, Schmidt GA, Wood LD, eds. Principles of Critical Care. McGraw-Hill;1998:579-595.
- Tuxen DV. Permissive hypercapnic ventilation. Am J Respir Crit Care Med 1994;150:870-874.
- Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis 1984;129:385-387.
- Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) vs. nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev 2013;2013:CD000052.
- Zorc JJ, Scarfone RJ, Li Y, et al. Scheduled follow-up after a pediatric emergency department visit for asthma: A randomized trial. Pediatrics 2003;111:495-502.
- Andrews AL, Teufel II RJ, Basco Jr WT. Low rates of controller medication initiation and outpatient follow-up after emergency department visits for asthma. J Pediatr 2012;160:325-330.
- Andrews AL, Russell WS, Titus MO, et al. Quality improvement methods improve inhaled corticosteroid prescribing in the emergency department. J Asthma 2014;51:737-742.
- Leo HL. Predictors of primary care follow-up after a pediatric emergency visit for asthma. Pediatrics 2006;118(Suppl 1):S36.
- Sampayo EM, McLoughlin RJ, Tsevdos D, et al. Pediatricians support initiation of asthma controller medications in the emergency department: A national survey. Pediatr Emerg Care 2015;31:545-550.
- Dawood FS, Kamimoto L, D’Mello TA, et al. Children with asthma hospitalized with seasonal or pandemic influenza, 2003-2009. Pediatrics 2011;128:e27-e32.
- Centers for Disease Control and Prevention. Vaccination coverage among persons with asthma — United States, 2010-2011 influenza season. MMWR 2013;62:973-978.
