Management of Problem Wounds in the Emergency Department
AUTHORS
Kinjal N. Sethuraman, MD, MPH, Assistant Professor, Department of Emergency Medicine, University of Maryland, Baltimore
Michele Callahan, MD, Adjunct Assistant Professor Department of Emergency Medicine, University of Maryland, Baltimore
Jacob Wynes, DPM, Assistant Professor of Orthopaedics, University of Maryland, Baltimore
PEER REVIEWER
Steven M. Winograd, MD, FACEP, Assistant Clinical Professor of Emergency Medicine, Mt. Sinai Medical School, New York City
EXECUTIVE SUMMARY
- Evaluate arterial perfusion in all chronic wounds: Determine the presence or absence of pulses by palpation or Doppler exam, and calculate the ankle-brachial index in lower extremity wounds.
- The ideal wound dressing will provide moisture to dry wounds and absorb excess moisture from wet wounds.
- Targeted sharp debridement — using scissors or a scalpel — is preferred to nonspecific debridement by using wet-to-dry gauze.
- A swab wound culture is less accurate than a deep tissue culture, and a swab wound culture should be done from viable tissue after debridement and rinsing of the wound, not from the wound exudate or slough.
- Urgent hyperbaric oxygen therapy also may be useful for flap salvage in failures caused by both venous congestion and disruption to arterial flow.
Introduction
The skin is the largest organ in the human body, accounting for approximately 16% of total body weight.1 The main functions served by the skin include maintenance of body temperature, protection from the external environment, providing a barrier against microorganisms, prevention of water and electrolyte loss, and prevention of damage by ultraviolet light.1
Traumatic lacerations, burns, and other wounds account for about 4% of emergency department (ED) visits.2 Most acute wounds will heal with good topical care. However, some wounds, especially chronic ones occurring in impaired hosts, are more problematic.
To properly manage these types of wounds and optimize healing, there should be an evidence-based approach to wound care in the ED. Knowledge of basic and advanced wound care principles and techniques are paramount in providing appropriate, efficient, and cost-conscious care.
Wound Healing Process
There are four main phases of physiologic wound healing: hemostasis, inflammatory, proliferative, and remodeling.3 These are regulated by a unique group of cytokines and growth factors.4 In contrast to healthy, well-healing wounds that progress through each stage, chronic wounds are arrested in one of the stages of wound healing. Patients with chronic wounds may present to the ED after wound neglect, inappropriate care, or misdiagnosis. Wounds can be further classified as healthy if they have non-friable tissue, whereas a wound that appears unhealthy by definition exhibits signs of a dark red discoloration and dehydration with a dull surface.5 A chronic wound is a wound that fails to progress over a 30-day period for various reasons, including underlying diabetes, prior radiation to the area, older age, inflammatory or immune dysfunction, or recurrent trauma.6 In contrast to the commonly accepted hypothesis of host-centered pathology, it is possible that surface bacteria, not host dysfunction, cause the chronicity and perpetual inflammation associated with chronic non-healing wounds.7 Factors that contribute to poor wound healing and chronic wounds are summarized in Table 1.
Table 1. Factors that Contribute to Chronic Wounds |
|
Basics of Wound Care
The basics of good wound care include debridement of devitalized tissue, control of infection and re-colonization of biofilm, maintenance of a well-
vascularized area, and proper moisture balance with minimal edema.8 Wound size, wound duration, wound grade, and percent healing at four weeks have been associated with a prognosis for healing at 12 and 20 weeks.9 These tenets can all be addressed in the ED.
Debridement
Debridement is the removal of surface debris, slough, and infected matter from the wound bed, yielding clean, viable tissue and enhancing the likelihood of healing.10 The presence of dead tissue in a wound provides a nidus for bacterial infection. There are several methods of debridement, including bedside sharp debridement (using a scalpel or scissors), surgical consult and operative intervention, enzymatic, and the use of medical-grade larvae. Sharp debridement may be the most feasible for emergency physicians (EPs). It involves stabilizing wound edges with forceps while gently removing devitalized tissue using scissors or a scalpel. Topical lidocaine can be applied to the wound for 15 to 30 minutes prior to debridement for pain control.
After debridement has occurred, there should be a reduction in wound exudate, with oozing of blood and the appearance of a healthy wound bed. Mechanical, indiscriminatory debridement such as a wet-to-dry dressing is generally not recommended and has fallen out of favor. Debridement can play an important role in the removal of biofilms and control of infection. Debridement also will stimulate wound healing.11
Infection Control
The presence of infection within a wound leads to failure to heal, increased pain, morbidity, and mortality.12 It is important to reassess wounds frequently to determine if there is colonization of bacteria within the wound. The management of wound infection varies from topical agents to systemic antibiotics.13
If a wound is believed to be infected after removal of necrotic tissue, the use of topical antimicrobials can be beneficial when used for up to a maximum of two weeks.13 Some topical agents, such as those containing povidone iodine, may not be cytotoxic to living cells working to form granulation tissue as previously thought.14 Based on recent studies, heavy metal antimicrobials (such as silver) may precipitate resistance of organisms, and the evidence supports antimicrobial activity prior to overt cytotoxicity of fibroblasts. Povidone iodine has been demonstrated to slow epithelialization but promote rapid neovascularization and no impediment to healing in clean wounds.15
It should be recognized that the wound develops increased susceptibility to bacterial colonization at 24-48 hours post debridement, and chronic wounds may develop significant tolerance to antimicrobial agents, disinfectants, and host immune defenses. Use of topical antimicrobials in conjunction with thorough debridement can suppress reformation of harmful biofilm.16,17 Frequent (every 24 to 48 hours) assessment of the wound while using these topical antimicrobials can guide therapy. Referral to a wound care center or surgical staff will be necessary. In some cases, or in low-resource settings, patients may need to follow-up directly in the ED.
Adequate Circulation
Insufficient blood flow is an important cause of delayed or failed wound healing.18 Patients with systemic diseases, such as peripheral vascular disease, diabetes, and tobacco smokers, are at increased risk for wound development as well as impaired wound healing. Oxygenation and perfusion to a wound are the most important factors in promoting wound healing. A mean arterial pressure less than 80 mmHg is predictive of poor wound healing.18
Causes of impaired blood flow may include diseases that impair microcirculation and conditions that incite a sympathetic response causing peripheral vasoconstriction.19 The presence of oxygen in a wound bed allows for oxidative killing of bacteria, collagen synthesis, angiogenesis, and epithelialization.20 Arterial perfusion to a limb can be assessed using noninvasive vascular studies in the form of multi-segmental ankle-brachial indices and either pulsed volume recording (PVR) or lower extremity arterial Doppler, particularly if pulses are diminished/absent or if prior vascular intervention had been performed. Arterial brachial index less than 0.5 is prognostic for poor wound healing and higher risk of major amputation.21 Timely, but not necessarily emergent, vascular surgery consultation can offer treatment options to immediately provide reperfusion, or to offer minimally invasive diagnostic and therapeutic modalities to address macrovascular perfusion deficits.
Appropriate Wound Environment
Studies have proven that moist wounds heal more efficiently than wounds allowed to dry out.22 However, the goal is to avoid excessive wound fluid while still maintaining a moist environment. Wound exudate contains water, electrolytes, nutrients, inflammatory mediators, matrix metalloproteinases, growth factors, and waste products. A moist environment promotes granulation, autolytic processes, angiogenesis, and migration of epidermal cells across the wound base.22
The quality of the exudate can guide management, and the volume can function as a measure of contamination status. For example, in dry wounds, the use of moist saline gauze may be appropriate. In wounds with significant exudate, hydrocolloids and alginates may dry the area while maintaining some moisture. The goal in the ED is to identify wounds that are “too wet” or “too dry” and prescribe the appropriate wound care therapy that will result in a moist “not too wet, not too dry” wound.22
Emergency Department History and Examination
In addition to documenting the patient’s risk factors for poor wound healing, obtaining a comprehensive wound history and physical exam are essential skills for emergency physicians. (See Table 2.) Photographs with size comparison (ruler or a common object) that can be embedded into electronic medical records will help monitor wound progress. This is especially important for patients who frequently return to the ED or can follow up as an outpatient with primary care or a specialist.
Table 2. Elements of Wound History and Physical Exam |
|
Wound Infections
All wounds are colonized with microorganisms, but the presence of infection often is defined by two or more signs of inflammation or purulence.23 Classic signs of infection include warmth, erythema, inflammation, and pain. These may not always be present in patients with chronic medical conditions such as diabetes because of their predisposition for peripheral neuropathy and a poor vascular supply. It is also important to probe for systemic symptoms of infection such as fevers, fatigue, nausea, vomiting, or malaise.
The use of prophylactic antibiotics for prevention of wound infection is controversial because local resistance can develop if an antibiotic is administered below the known bacterial minimum inhibitory concentration.24 Wounds for which prophylactic antibiotics are acceptable include animal or human bites, open fractures, grossly contaminated wounds, and puncture wounds.25 If there is concern about development of wound infection, follow-up within 24-48 hours is advised.
If infection is suspected, the wound may be cultured to identify the offending organism(s). The gold standard for wound culture is a tissue biopsy. This involves removing living tissue from the wound for examination. Swab cultures of the wound surface (or fluid from the wound) can be useful for superficial wounds but may only reflect surface contamination or colonization and are less sensitive at identifying gram-negative organisms and all organisms growing in deeper wounds.26 These cultures are less reliable but easier to obtain. Concordance of superficial wound culture and tissue culture is only 22.5%.27
If obtaining a swab culture, it should be performed after debridement and rinsing of the wound and, thus, should not be a reflex nursing order. The swab should be done only on viable tissue, not the exudate or slough of the wound. Results of these cultures may be useful in antibiotic selection if performed appropriately.
If there is concern for osteomyelitis, the gold standard is a bone biopsy.27 However, the diagnosis of osteomyelitis is based on a preponderance of available data and should not be based solely on one diagnostic modality. Advanced imaging (beyond plain film radiographs), such as magnetic resonance imaging (MRI) or Ceretec/Indium-labeled nuclear medicine imaging, should be correlated with serologic markers (erythrocyte sedimentation rate [ESR], C-reactive peptide [CRP]) and clinical evaluation.27,28 Consultation with a surgical service for appropriate operative management of infected bone is warranted.
Wounds Commonly Seen in the Emergency Department
Diabetic Ulcers
Diabetes is a common disease in the United States and affects an estimated 8.7% of the adult population.29 Lower extremity amputations occur in 5.6/1,000 diabetic patients.30 Diabetic foot infections are a common problem seen in the ED as the incidence of diabetes continues to rise. In addition, diabetic foot complications are the leading cause for diabetes-related hospitalization and lower extremity amputation.31 The cost of amputation is high both in monetary terms and in terms of quality of life.31 These wounds most commonly begin as a skin compromise with subdermal hemorrhage and often are the result of peripheral neuropathy in conjunction with trauma and abnormal biomechanics that may not have been noticed immediately by the patient. Additional risk factors, such as impaired wound healing and patient comorbidities, contribute to poor healing. Most diabetic foot infections are polymicrobial, including gram-positive cocci and staphylococci as well as gram-negatives (seen more often in chronic wounds) and anaerobes (particularly in necrotic tissue).32 Diabetic foot ulcers with no evidence of surrounding soft tissue or bone infection do not require antibiotic therapy.
In examining a patient with a diabetic foot wound, the patient’s extremity and wound should be evaluated for signs of arterial ischemia, venous insufficiency, and alterations in sensation. Plain films may be helpful to evaluate for osseous deformity, prior surgical procedures, and cortical irregularities or other radiographic signs of osteomyelitis, if concern exists. If bone can be palpated, osteomyelitis can be assumed, and antibiotics should be initiated without delay. (See Figures 1-3 for examples of diabetic foot ulcers.)
Figure 1. Diabetic Foot Ulcer at the Plantar Base of First Metatarsal Joint |
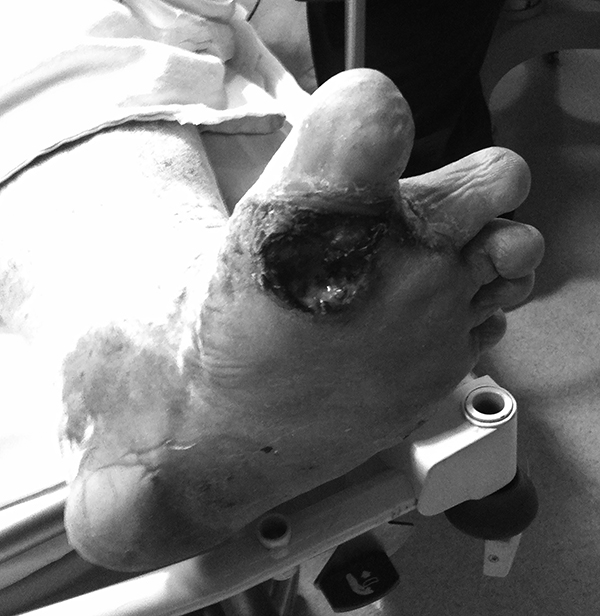 |
Photo courtesy of Jacob Wynes, DPM. |
Figure 2. Diabetes Mellitus Ulcer with Infection; Fat and Muscle Exposed |
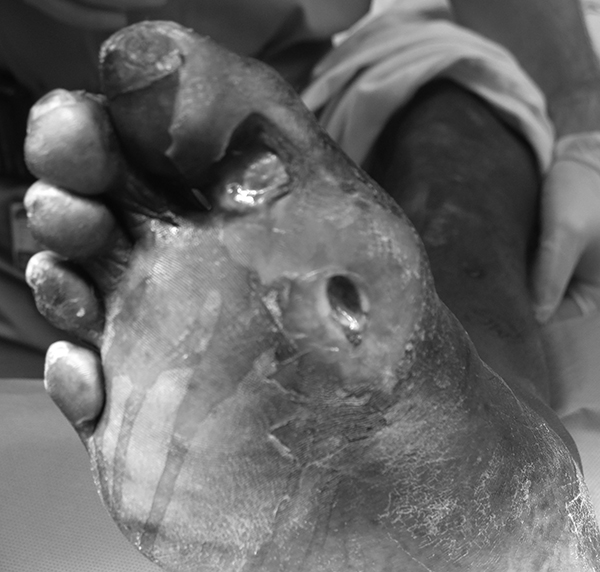 |
Photo courtesy of Jacob Wynes, DPM. |
Figure 3. Plantar Diabetes Mellitus Ulcer with Necrosis of Skin |
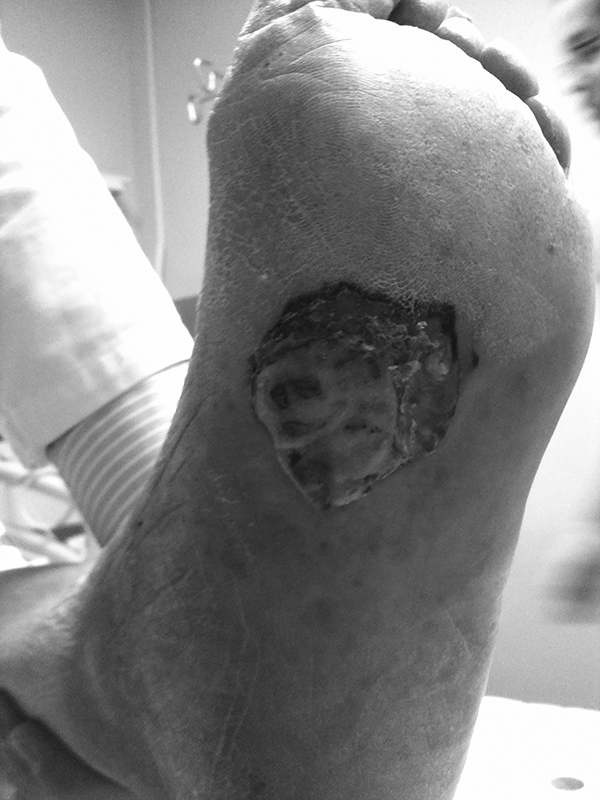 |
Photo courtesy of Jacob Wynes, DPM. |
Appropriate wound care should include debridement, “off-loading” of any areas to redistribute pressure away from the wound, and appropriate selection of dressings to allow for a biofilm-free, moist environment.33 Patients who may require hospitalization include those with signs of moderate to severe infection, lack of home support or ability to provide adequate wound care to themselves, and failure to respond to outpatient therapies. Multidisciplinary evaluation has been shown to lower the amputation rate dramatically.34
It is not necessary to admit patients with a diabetic wound if follow-up can be established and a short-term wound care plan can be established by the EP. At minimum, a good skin exam should be performed in all diabetic patients who are seen in the emergency department. Telemedicine has been shown to improve wound outcomes and decrease amputation rates in diabetic patients. Patients seen in the ED may benefit from initiation of a tele-relationship with a wound care provider.35
Frostbite
Frostbite is a cold-induced injury to tissue caused by endothelial damage. The injury to tissue is related to cold-induced cell death (often from crystal formation), as well as inflammation leading to tissue ischemia and necrosis.36 Areas on the body that are most commonly affected include the ears, nose, fingers, toes, and cheeks. On exam, the skin may appear gray-white, hard to the touch, and may be insensate. If the tissue is already necrotic, it may appear black or have noticeable eschar. During the thawing process, the development of blisters and bullae may occur. Management of frostbite can begin in the prehospital setting with removing wet clothing and getting the patient to a warm environment. On arrival to the ED, rapid rewarming can be accomplished by immersion into warm water. A water temperature of 37-39°C is most appropriate. Refreezing should be avoided.
Once rewarming has occurred, there should be a focus on appropriate wound care for the affected areas.37,38 This involves non-adherent dressings and elevation of the area to reduce edema. Aspirin or ibuprofen may be administered. Thrombolytic therapy has been studied and may be helpful to prevent amputation when administered in the first 24 hours of injury.39 Cases have been reported of hyperbaric oxygen therapy (HBOT) use in frostbite for tissue preservation and demarcation of necrotic tissue even when HBOT is delayed.40 Surgical consultation is appropriate in cases that may require angiography, frequent hydrotherapy, debridement, or amputation. Tetanus immunization status should be assessed. The decision about whether to debride blisters is a controversial topic without any clear consensus.
Pressure Injury
Pressure injuries are caused by pressure-induced necrosis with resultant damage to the skin and underlying structures, often over bony prominences where pressure and shear force occur. These compressive forces lead to local tissue ischemia and obstruction of lymphatic channels. Local edema and inflammation can occur and lead to further tissue necrosis. In the United States, millions of pressure ulcers are treated yearly, costing an average of $26 billion per year.41 Risk factors for pressure ulcers are widely variable, but include immobilization, advanced age, obesity, congestive heart failure, and poor nutritional status.42,43
Management and prevention of pressure ulcers require a multidisciplinary approach. Risk factors should be monitored for and addressed as appropriate. When present, these wounds may be painful (if sensory nerves are intact) or painless. They may be edematous, erythematous, and hyperemic, or cool with evidence of necrosis. Once identified, pressure ulcers often are managed conservatively, although up to 27% may required flap closure.44
Wound care for patients with pressure ulcers involves protecting the surrounding skin, dressing the wound bed, debriding or controlling infection if necessary, minimizing pressure and shear forces, and educating the patient and caregivers. If ulcers become extensive, surgical interventions, such as more intense debridement or flap closure, may be beneficial.45
The most important intervention for management for pressure ulcers is prevention. The nature of emergency medicine evaluation and the often long ED stays on suboptimal mattresses can lead to and/or exacerbate pressure ulcers in immobile patients. Frequent turning while patients are boarding in the ED also will decrease the risk of pressure ulcer formation. With limited nursing time available for turning patients at specific intervals, patients who are unable to self-adjust their weight in the stretcher and require long ED stays or are boarding in the ED may benefit from air mattresses on hospital beds rather than remaining on stretchers. Even with all prevention methods in place, for some higher risk patients, pressure injury may be unavoidable.46
Venous Stasis
Venous insufficiency ulcers are the most common type of leg ulcer, commonly believed to account for 70% to 90% of all leg ulcers.47 The presence of venous hypertension (related to vein dysfunction, calf muscle pump failure, or a combination of these) and endothelial damage is required for development of these ulcerations.48 Venous hypertension and distention of tissues leads to increased vascular congestion and resultant hypoxia causing further endothelial damage, ultimately leading to skin and tissue breakdown. This leads to the formation of venous stasis ulcers, often found on the medial lower leg and in areas of trauma. The pain associated with these ulcers is controlled by elevation and compression. Venous stasis ulcers may appear irregularly shaped and are found in skin areas with edema, dermatitis, and hemosiderin deposition. (See Figures 4-7.)
Figure 4. Venous Stasis Ulcer on Lower Leg with Granulation Tissue After Compression Dressing |
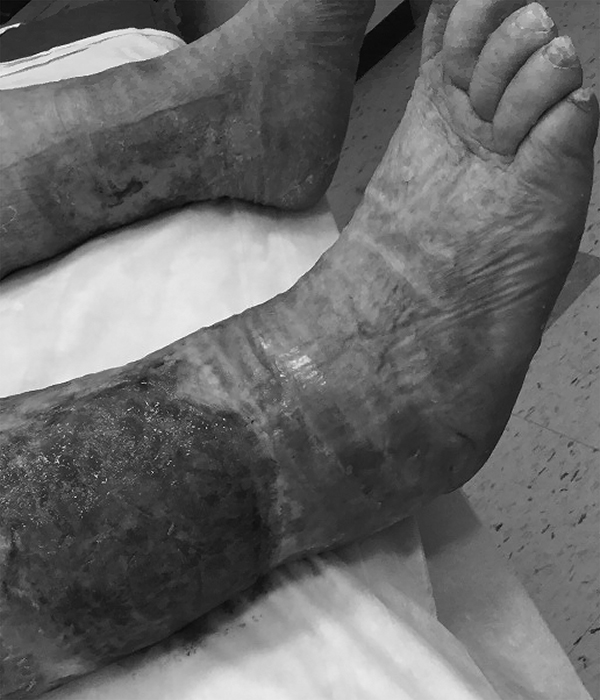 |
Photo courtesy of Zachary Gaskill, DO. |
Figure 5. Severe Venous Stasis with Hemosiderin Deposition |
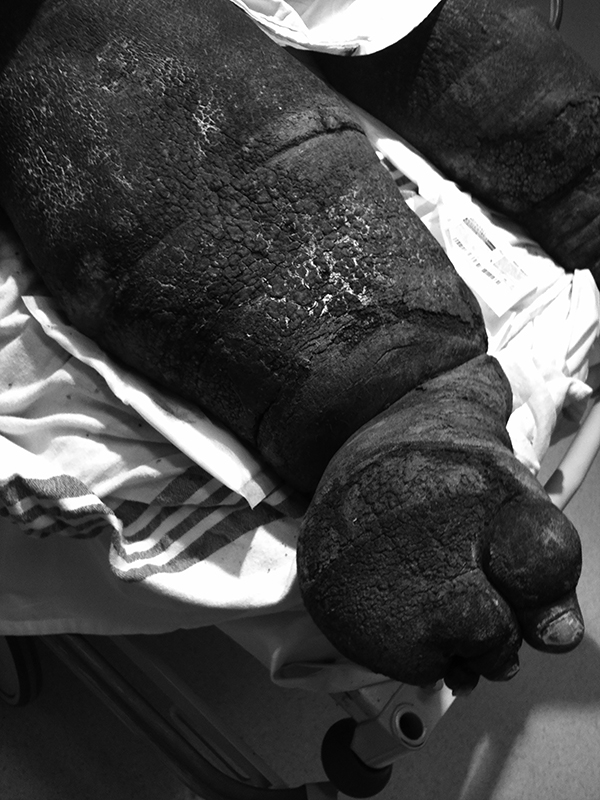 |
Photo courtesy of Jacob Wynes, DPM. |
Figure 6. Venous Ulcer with Necrosis, Exudate, Maceration |
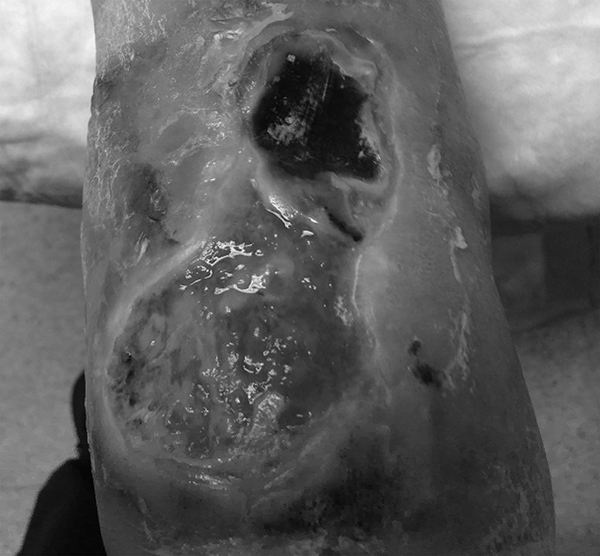 |
Photo courtesy of Zachary Gaskill, DO. |
Figure 7. Ulcer with Surrounding Maceration, Tunneling |
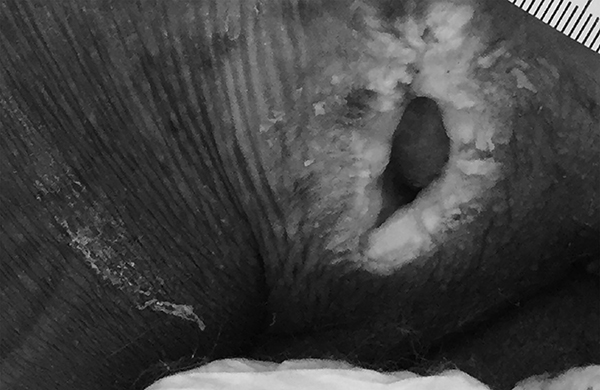 |
Photo courtesy of Zachary Gaskill, DO. |
Treatment often relies on using compression and elevation to control edema, protecting the legs and feet from trauma, and controlling other medical conditions, such as diabetes and hyperlipidemia. Accurate diagnosis and biopsy are important to guide treatment since common differentials could include pyoderma gangrenosum or vasculitis, particularly if pathergy exists.
In the ED, treatment can start with light compression with an ACE bandage after dressing the wound with either absorbent dressing if the wound has significant discharge and is “too wet,” or Vaseline-impregnated gauze if the wound is too dry, and only if distal pulses are intact (palpable or audible on Doppler). Outpatient follow-up with a wound care center should occur within one week. If the patient or caregiver can change the dressing correctly, a telemedicine visit may be appropriate.
Arterial Insufficiency and Ulceration
Peripheral arterial disease (PAD) is an insidious process that affects up to 3% to 10% of Americans.49 Arterial insufficiency and ulceration occur when inadequate arterial flow reaches tissues, causing ischemia and necrosis. (See Figure 8.) This leads to the arterial ulcers seen on exam. Most commonly, arterial ulcers are related to atherosclerosis and resultant luminal narrowing of the vessel. Often, this is a more chronic process; however, there can be acute ischemia in the setting of embolic events.
Figure 8. Arterial Embolism Causing Necrosis of First Metatarsal |
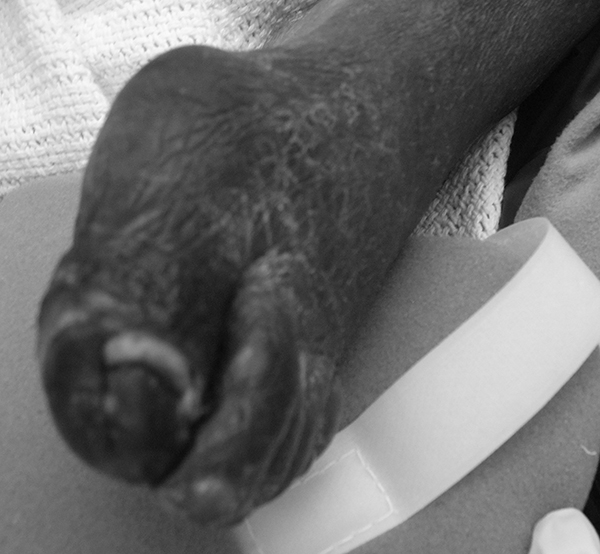 |
Photo courtesy of Jacob Wynes, DPM. |
In evaluating patients for arterial ulcerations, check the most distal points of perfusion. This requires removing patients’ socks and evaluating their toes and pressure points (such as the heel). During the initial evaluation, the EP should measure the ankle-brachial index to screen for PAD and narrow the underlying cause for an ulcer.50 Arterial insufficiency ulcers often will appear pale and dry, with clear demarcations, as opposed to the edematous and erythematous regions seen in association with venous ulcerations. Typically, patients also will have findings of long-standing arterial insufficiency, such as shiny skin and hair loss. Patients will describe claudication and pain that often is cramping in nature.51
Risk factors include hyperlipidemia, smoking, diabetes, hypertension, and advancing age. Risk factor modification and appropriate wound care are required. Patients also should be referred to a vascular surgeon for revascularization and definitive management. Emergent vascular surgery consultation is warranted if acute ischemia is suspected.
Flap Failures
Flap surgery is a technique in reconstructive surgery where tissue is lifted from a donor site and moved to a recipient site with an intact blood supply. Flap failures occur most often due to vascular obstruction such as venous or arterial congestion. Smoking, diabetes, hypertension, elevated body mass index, and surgery in an irradiated field increase risk of flap failure.52 Patients likely will be seen by their surgeon for this complication. Some patients may arrive to the ED when the complication occurs or may be referred to the ED by a surgeon for further management.
The first step in management is the recognition that failure is occurring, using both clinical observation as well as tests for pin-prick sensation, measurement of temperature, and the use of surface Doppler. Rapid surgical exploration and reanastomosis may be needed to preserve the flap. Although unlikely to be initiated in the ED, leech therapy for venous congestion causing flap failure has been used successfully.53 Urgent HBOT also may be useful for flap salvage in failures caused by both venous congestion and disruption to arterial flow.53 HBOT can maximize the viability of the flap or graft by promoting angiogenesis and decreasing edema. To be most effective, HBOT should be initiated as soon as possible once signs of flap failure appear.
Autoimmune Wounds
Slow-healing wounds are a known complication of many autoimmune diseases, such as rheumatoid arthritis and scleroderma. These wounds may occur in the setting of vasculitis and inflammation affecting the small blood vessels in the skin. In addition, there are wounds of unclear etiology, such as pyoderma gangrenosum, an auto-inflammatory condition of ulcerative cutaneous lesions.
The management of these skin wounds involves identifying the underlying cause, modifying risk factors, providing local wound care, applying appropriate dressings, and avoiding infection. In the ED, patients may present with the first manifestation of autoimmune diseases in the form of ulcerations. A thorough history and referral for biopsy may be offered. Standard wound care and infection mitigation strategies may be initiated in the ED. In addition, the use of HBOT is a helpful adjunct for wound healing in these autoimmune diseases, likely due to its anti-inflammatory effect. After an initial work-up for an underlying cause, patients may be referred for HBOT.
Selection of Appropriate Wound Care Dressing
Specialty wound care dressings may not be readily available in the ED, but they can be requested from inpatient supply areas. Dressing selection for wounds can have a significant impact on healing, and ED staff should be familiar with them. (See Table 3.) The function of a wound dressing is to create an appropriately moist environment. If the wound is too wet, the dressing should absorb excess exudate. If the wound is too dry, the dressing should provide moisture to the wound bed.54
Table 3. Wound Dressings | |||||
Dressing Type | Brand Names | Characteristics | Uses | Advantages | Disadvantages |
Gauze | Kerlix | Thin fabric with a loose, open weave | Inexpensive, readily available | May remove viable tissue if taken off forcefully when dry | |
Impregnated gauze | Adaptic, Xeroform | Gauze dressings impregnated with petrolatum or zinc | Burn wounds | Minimal trauma with removal; more occlusive and allow for moisture retention within wound | Requires additional layer of dressing on top (such as dry gauze) to secure |
Films | BIOCLUSIVE, Tegaderm, Cutifilm | Thin sheets of polyurethane; impermeable to water and bacteria but allow CO2 and O2 to permeate | Skin tears, donor graft sites, skin abrasions | Retain moisture, reduce friction; ability to visualize wound bed through dressing | Not useful in wounds with heavy exudates or infected wounds |
Hydrogels | Iodosorb, Aquasorb, Elta Hydrogel | Water- or glycerin-based gels | Skin tears, blisters, donor graft sites, pressure ulcers, radiation or thermal burns, surgical sites | Add moisture to dry wounds; may offer pain relief/cooling sensation; facilitate autolytic debridement | Provide little barrier to infection; non-adherent and require additional dressing on top to secure |
Foams | ALLEVYN, | Polyurethane foams | Burn wounds, graft and donor sites, ulcers (pressure, venous insufficiency, and diabetic) | Retain moisture, provide thermal insulation, provide cushioning, absorb moderate amounts of exudate | Not ideal for wounds with eschar, dry wounds, or wounds on areas of friction (such as the heel) |
Hydrocolloids | DuoDERM, Comfeel | Hydrophilic colloid particles with film or foam adhesive backing | Pressure ulcers, minor burns, venous insufficiency ulcers; ideal for partial- or full-thickness wounds | Absorb exudate; thermally insulate wounds; act as impermeable barriers to water, bacteria, and oxygen; lower rates of infection | Not ideal for use in areas with high friction, wounds with heavy exudate or bleeding, dry wounds, or actively infected wounds |
Alginates | CURASORB, ALGISITE M | Brown seaweed extract that forms a hydrophilic gel | Wounds with moderate-to-heavy drainage, partial- or full-thickness wounds | Provide moisture; trap bacteria; highly permeable | Non-occlusive and require second dressing layer on top; not for use on top of tendon or bone as they can lead to dessication |
Many EPs still may be using wet-to-dry gauze dressings. However, this previously popular technique has fallen out of favor. In fact, use of wet-to-dry dressings can lead to increased pain and delayed healing if the dressing adheres to the wound bed and causes trauma on removal. If patient is admitted to the hospital and frequent wet-to-dry dressing changes that do not damage the wound bed can be done, then this dressing type may be appropriate.
Wound dressings serve additional important functions, including providing a barrier against infection and thermal injury, removing debris, and decreasing pain by protecting exposed nerves.54 The following is a summary of dressing types that EPs may encounter.
Gauze Dressings
Gauze dressings are useful as the dressing of choice for select types of wounds. If there are daily dressing changes, gauze is the most economical choice. It also is useful for very large or irregularly shaped wounds because it is inexpensive and can be shaped appropriately and secured in place easily. Keep in mind that gauze, once dry, may require force to remove from the wound bed. This can lead to nonselective removal of healthy new tissue along with the rest of the debris and, therefore, it must be changed gently and frequently. (See Figure 9.)
Figure 9. Dry Gauze Attached to Diabetic Wound Causing Nonspecific Debridement |
 |
Photo courtesy of Jacob Wynes, DPM. |
Impregnated Gauze Dressings
The use of impregnated gauze dressings, such as those with petrolatum or zinc, allows for a less traumatic removal. These dressings also are more occlusive, allowing for retention of moisture in the wound. Impregnated gauze is particularly useful for burn wounds, often in conjunction with silver sulfadiazine or other topical antimicrobials. There are few data on silver sulfadiazine for burns, limited to a few animal studies.55 These impregnated gauzes often require a secondary layer over the top, typically dry gauze. Xeroform gauze is unique in that it is impervious and may carry the added antibacterial benefit to some organisms through its incorporation with bismuth, but this benefit may be minimal compared to direct application of bismuth alone.56
Films
Semipermeable film dressings are thin, flexible sheets of polyurethane with an adhesive backing that are routinely used in American emergency departments for securing intravenous lines. These sheets are impermeable to water and bacteria, but permeable to carbon dioxide and oxygen. The benefits of these include retention of moisture, reduced friction, and ability to visualize the wound bed through the dressing. These are not appropriate for use with wounds that have moderate to heavy exudates or infected wounds. Common uses of film dressings for wounds include skin tears, donor graft sites, and abrasions.57
Hydrogels
Hydrogels are often > 80% water-based or glycerin-based and are useful for adding moisture to dry wounds. Hydrogel formulation includes a gel formulation in a tube, or impregnated into sheets or gauze pads, making it versatile. They can absorb small amounts of fluid by swelling, and also are able to donate moisture to dry wounds. Typically, hydrogels are indicated for minimally draining wounds or necrotic wounds.
Hydrogels generally are less impermeable than films and do less to provide a barrier against bacterial infection. When placed on a wound, hydrogels often provide pain relief and a cooling effect. They also facilitate autolytic debridement of wounds. Since hydrogels are non-adherent, they require a secondary dressing on top to secure them in place. Common uses of hydrogels include skin tears, blisters, donor graft sites, radiation and thermal burns, pressure ulcers, and surgery sites.54
Foams
Foam wound dressings are created with polyurethane foam, with a hydrophilic wound side and a hydrophobic outside. These often are very good at retaining moisture, providing thermal insulation, cushioning, and absorbing a moderate amount of exudate. These are most useful on burns, graft and donor sites, pressure ulcers, and diabetic and venous insufficiency ulcers. Polyvinyl alcohol combined with methylene blue and gentian violet foam (Hydrofera Blue Classic dressing®) has been successful in treating localized infection, particularly if patients are unable to follow up for routine office or clinic visits. Foams are used to cover a necrotic wound and will interact with the patient’s own body heat, causing autolytic debridement of the wound. Foams are less useful for wounds that are dry, covered in eschar, or areas of friction such as the heel.54,58
Hydrocolloids
Hydrocolloid dressings are made of hydrophilic colloid particles with a film or foam adhesive backing. These work by absorbing exudate and swelling into a gel-like substance. They act to thermally insulate wounds, and as impermeable barriers to water, oxygen, and bacteria. This leads them to have lower rates of infection compared to wounds with dressings such as gauze or foams. Hydrocolloids are used commonly for wounds that are partial- or full-thickness; they are used most often for pressure ulcers, minor burns, and venous insufficiency ulcers. They should be avoided in areas of high friction (because they can roll off), wounds with heavy exudate or active bleeding, dry wounds, or actively infected wounds.54,59,60
Alginates
Alginates are made of ingredients extracted from brown seaweed. When placed into a wound, they form a hydrophilic gel that provides moisture and acts to trap bacteria. Alginates are highly permeable and nonocclusive, often requiring a second layer of dressing on top (such as gauze). They are available as sheets to lay on top of a wound bed, ropes to fill undermining areas, and alginate-tipped applicators for probing and filling wound cavities.
Alginates should be used for wounds with moderate to heavy drainage, and partial- or full-thickness wounds such as pressure ulcers. They may be used on infected wounds but should be replaced daily when soiled. Otherwise, these dressings can be maintained three days at a time. They should not be placed on wounds with exposed tendon or bone, as this may lead to desiccation of the wound. Alginates are very effective in debriding necrotic tissue, and some formulations possess a silver coating to reduce time to healing.54
Deciding When to Admit to the Hospital
The decision to admit a patient with a wound can be difficult without prior knowledge of how that wound has looked or been treated in the past. Often, the first step of this decision is based on clinical exam. Is there significant erythema, warmth, friable tissue, or copious and/or malodorous discharge? Are there systemic signs or symptoms such as fevers or generalized weakness? These can indicate that outpatient management is not sufficient. Often, there are patient factors that also play a role. For example, does the patient have poor compliance or lack a support system? If patients are unable to use resources as an outpatient, they may require closer monitoring in an inpatient setting. If there is a need for operative debridement or aggressive vascular intervention, this would also necessitate inpatient admission.
In patients who lack any warning signs and whose wounds appear to be healing well with their current regimen, it is likely safe to perform a trial of continued outpatient treatment. On discharge, it is important to ensure that patients have close follow-up for a wound check within several days of their visit to the emergency department. If this occurs in a resource-limited area, patients may need to return to the ED or urgent care for timely wound checks. Patients or caretakers can take photographs of the wound’s progression and provide the images at the outpatient follow-up visit. Telemedicine visits can be used for wound care visits with patients who can maneuver the technology.
Deciding When to Involve Specialists
On occasion, consultation of wound care specialties such as podiatry, vascular surgery, and hyperbaric medicine may be required from the ED.
Vascular Surgery
In general, patients with lower extremity ulcerations should be assessed for arterial disease, beginning with a pulse exam. Measurement of the ankle-brachial index (a value of < 0.9 is considered abnormal) also can provide information about arterial insufficiency. Unfortunately, the natural history of arterial disease is to progress and potentially lead to limb loss, so involvement of vascular surgery early in the course is paramount. Arterial disease often requires revascularization at some point, even with adequate and appropriate wound care. Patients with evidence of critical limb ischemia (sudden onset of resting pain, pallor, pulselessness, paralysis, paresthesia, or new skin necrosis) should be referred emergently for vascular intervention. Compressive dressing for peripheral edema or venous stasis ulcers should not be placed on the legs of patients who do not have a palpable pulse.
Hyperbaric Medicine
Hyperbaric medicine is a subspecialty of emergency medicine that also has significant crossover into outpatient medicine. Wound management is one area where this intersection occurs. There are several wound-related emergent indications where consultation with a hyperbaric facility in conjunction with surgery is beneficial. These include necrotizing soft tissue infections, gas gangrene, crush and compartment syndrome, and flap failure. Additional non-emergent conditions that benefit from HBOT include refractory osteomyelitis, delayed radiation injury, and chronic wounds.
HBOT acts by increasing the oxygen concentration gradient, which may reduce bacterial growth and enhance processes integral to wound healing: angiogenesis, collagen synthesis, granulation tissue formation, epithelialization, and wound contraction.
Podiatric Surgery
Obtaining a consultation from podiatric surgery is appropriate in distal lower extremity wounds that require partial functional amputations, extensive debridement, and surgical reconstruction of foot and ankle deformity. Based on their ability to manage all aspects of foot and ankle limb salvage, podiatric surgeons also should be involved with the outpatient management and monitoring of chronic foot wounds. Referral to a podiatrist within one week is appropriate for patients with lower extremity wounds that do not require urgent intervention.
Conclusion
Because of the large volume of acute and chronic wounds that present to the emergency department each year, it is imperative that EPs become familiar with proper management of these wounds to prevent progression of the wound and potentially avoid costly admissions. Outpatient close follow-up is critical to determine the underlying cause of a wound and avoid a delay in diagnoses. Taking a thoughtful approach to wound care and working toward optimizing wound healing may improve patient outcomes, prevent admission, and optimize resources.
REFERENCES
- Leider M. On the weight of the skin. J Invest Dermatol 1949;12:187-91.
- Centers for Disease Control and Prevention. National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. Updated Nov. 10, 2020. http://www.cdc.gov/nchs/fastats/emergency-department.htm.
- Sorg H, Tilkorn DJ, Hager S, et al. Skin wound healing: An update on the current knowledge and concepts. Eur Surg Res 2017;58:81-94.
- Qing C. The molecular biology in wound healing & non-healing wound. Chin J Traumatol 2017;20:189-193.
- Flanagan, M. The physiology of wound healing. J Wound Care 2000;9:299-300.
- Hartoch RS, McManus JG, Knapp S, Buettner MF. Emergency management of chronic wounds. Emerg Med Clin North Am 2007;25:203-221.
- Schultz GS, Sibbald RG, Falanga V, et al. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen 2003;11(Suppl 1):S1-S28.
- Baranoski S, Ayello EA. Wound Care Essentials: Practice Principles, 2nd edition. Lippincott, Williams & Wilkins; 2008.
- Sheehan P, Jones P, Caselli A, et al. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Diabetes Care 2003;26:1879-1882.
- McIntosh C, Kelly L. Importance of wound debridement in management of diabetic foot ulcers. Wound Essentials 2009;4:122-124.
- Anghel EL, DeFazio MV, Barker JC, et al. Current concepts in debridement: Science and strategies. Plast Reconstr Surg 2016;138(3 Suppl):82S-93S.
- Das SK, Yuan YF, Li MQ. Predictors of delayed wound healing after successful isolated below-the-knee endovascular intervention in patients with ischemic foot ulcers. J Vasc Surg 2018;67:1181-1190.
- Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis 2009;49:1541-1549.
- Bigliardi PL, Alsagoff SAL, El-Kafrawi HY, et al. Povidone iodine in wound healing: A review of current concepts and practices. Int J Surg 2017;44:260-268.
- Kjolseth D, Frank JM, Barker JH, et al. Comparison of the effects of commonly used wound agents on epithelialization and neovascularization. J Am Coll Surg 1194;179:305-312.
- Eming SA, Smola-Hess S, Kurschat P, et al. A novel property of povidone-iodine: Inhibition of excessive protease levels in chronic non-healing wounds. J Invest Dermatol 2006;126:2731-2733.
- Attinger C, Wolcott R. Clinically addressing biofilm in chronic wounds. Adv Wound Care (New Rochelle) 2012;1:127-132.
- Smollock W, Montenegro P, Czenis A, He Y. Hypoperfusion and wound healing: Another dimension of wound assessment. Adv Skin Wound Care 2018;31:72-77.
- Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg 1991;126:1131-1134.
- Schreml S, Szeimies RM, Prantl L, et al. Oxygen in acute and chronic wound healing. Br J Dermatol 2010;163:257-268.
- Brownrigg JR, Hinchliffe RJ, Apelqvist J, et al; International Working Group on the Diabetic Foot. Performance of prognostic markers in the prediction of wound healing or amputation among patients with foot ulcers in diabetes: A systematic review. Diabetes Metab Res Rev 2016;32 (Suppl 1):128-135.
- Hinman CD, Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature 1963;200:377-378.
- Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis 2012;54:e132-e173.
- Abbas M, Uçkay I, Lipsky BA. In diabetic foot infections antibiotics are to treat infection, not to heal wounds. Expert Opin Pharmacother 2015;16:821-832.
- Kramer A, Dissemond J, Kim S, et al. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol Physiol 2018;31:28-58.
- Huang Y, Cao Y, Zou M, et al. A comparison of tissue versus swab culturing of infected diabetic foot wounds. Int J Endocrinol 2016;2016:8198714.
- Senneville E, Melliez H, Beltrand E, et al. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. Clin Infect Dis 2006;42:57-62.
- Meyr AJ, Singh S, Zhang X, et al. Statistical reliability of bone biopsy for the diagnosis of diabetic foot osteomyelitis. J Foot Ankle Surg 2011;50:663-667.
- Centers for Disease Control and Prevention. Diagnosed diabetes. https://gis.cdc.gov/grasp/diabetes/DiabetesAtlas.html
- Gurney JK, Stanley J, York S, et al. Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia 2018;61:626-635.
- Raghav A, Khan ZA, Labala RK, et al. Financial burden of diabetic foot ulcers to world: A progressive topic to discuss always. Ther Adv Endocrinol Metab 2018;9:29-31.
- Ghotaslou R, Memar MY, Alizadeh N. Classification, microbiology and treatment of diabetic foot infections. J Wound Care 2018;27:434-441.
- Majid U, Argáez C. Off-loading devices for people with diabetic neuropathic foot ulcers: A rapid qualitative review. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2020 Jun 17.
- Rubio JA, Aragón-Sánchez J, Jiménez S, et al. Reducing major lower extremity amputations after the introduction of a multidisciplinary team for the diabetic foot. Int J Low Extrem Wounds 2014;13:22-26.
- Smith-Strøm H, Igland J, Østbye T, et al. The effect of telemedicine follow-up care on diabetes-related foot ulcers: A cluster-randomized controlled noninferiority trial. Diabetes Care 2018;41:96-103.
- Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: Pathogenesis and treatment. J Trauma 2000;48:171-178.
- Biem J, Koehncke N, Classen D, Dosman J. Out of the cold: Management of hypothermia and frostbite. CMAJ 2003;168:305.
- Handford C, Buxton P, Russell K, et al. Frostbite: A practical approach to hospital management. Extrem Physiol Med 2014;3:7.
- Wexler A, Zavala S. The use of thrombolytic therapy in the treatment of frostbite injury. J Burn Care Res 2017;38:e877-e881.
- Lansdorp CA, Roukema GR, Boonstra O, et al. Delayed treatment of frostbite with hyperbaric oxygen: A report of two cases. Undersea Hyperb Med 2017;44:365-369.
- Padula WV, Delarmente BA. The national cost of hospital-acquired pressure injuries in the United States. Int Wound J 2019;16:634-640.
- Karahan A, AAbbasoğlu A, Işık SA, et al. Factors affecting wound healing in individuals with pressure ulcers: A retrospective study. Ostomy Wound Manage 2018;64:32-39.
- Sethuraman KN, Leekha S, Adediran T, Roghmann MC. Association of pressure injury with body care activities in nursing homes. Wound Repair Regen 2020 doi: 10.1111/wrr.12861. [Online ahead of print].
- Diamond S, Moghaddas HS, Kaminski SS, et al. National outcomes after pressure ulcer closure: Inspiring surgery. Am Surg 2016;82:903-906.
- National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel and Pan Pacific Pressure Injury Alliance. Prevention and Treatment of Pressure Ulcers: Quick Reference Guide. Emily Haesler, ED. Cambridge Media: Osborne Park, Australia; 2014.
- Pittman J, Beeson T, Dillon J, et al. Hospital-acquired pressure injuries in critical and progressive care: Avoidable versus unavoidable. Am J Crit Care 2019;28:338-350.
- Phillips TJ, Dover JS. Leg ulcers. J Am Acad Dermatol 1991;25(6 Pt 1):965-987.
- Castro-Ferreira R, Cardoso R, Leite-Moreira A, Mansilha A. The role of endothelial dysfunction and inflammation in chronic venous disease. Ann Vasc Surg 2018;46:380-393.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation 2006;113:e463-e654.
- Dua A, Lee CJ. Epidemiology of peripheral arterial disease and critical limb ischemia. Tech Vasc Interv Radiol 2016;19:91-95.
- Sieggreen MY, Kline RA. Arterial insufficiency and ulceration — diagnosis and treatment options. Nurse Pract 2004;29:46-52.
- Las DE, de Jong T, Zuidam JM, et al. Identification of independent risk factors for flap failure: A retrospective analysis of 1530 free flaps for breast, head and neck and extremity reconstruction. J Plast Reconstr Aesthet Surg 2016;69:894-906.
- Kubo T, Yano K, Hosokawa K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery 2002;22:391-395.
- Baranoski S, Ayello EA. Wound Care Essentials: Practice Principles, 2nd ed. Lippincott, Williams & Wilkins; 2008.
- Miller AC, Rashid RM, Falzon L, et al. Silver sulfadiazine for the treatment of partial-thickness burns and venous stasis ulcers. J Am Acad Dermatol 2012;66:e159-e165.
- Barillo DJ, Barillo AR, Korn S, et al. The antimicrobial spectrum of Xeroform®. Burns 2017;43:1189-1194.
- 3M Health Care. Tegaderm film dressing package insert. https://multimedia.3m.com/mws/media/498994O/tegaderm-transparent-film-dressing-package-insert-english.pdf
- Smith + Nephew. Allevin. https://www.smith-nephew.com/key-products/advanced-wound-management/allevyn/
- ConvaTec. DuoDERM dressings. https://www.convatec.com/advanced-wound-care/duoderm-dressings/
- 3M Health Care. Tegaderm hydrocolloid dressing. https://multimedia.3m.com/mws/media/376798O/3m-tegaderm-hydrocolloid-dressing-sacral-design.pdf
