Arterial Ischemic Stroke: Mechanisms and Management
AUTHORS
Stephanie B. Rutrick, MD, Vascular Neurology Fellow, Department of Neurology, Weill Cornell Medical College, New York
Alan Z. Segal, MD, Associate Professor of Clinical Neurology, Department of Neurology, Weill Cornell Medical College, New York
PEER REVIEWER
Glen D. Solomon, MD, FACP, Professor and Chair, Department of Internal Medicine, Wright State University Boonshoft School of Medicine, Dayton, OH
EXECUTIVE SUMMARY
- Stroke can be divided into five primary etiologies: large-artery atherosclerosis, cardioembolism, small-vessel occlusion, stroke of other determined etiology, and stroke of undetermined etiology. Workup of the stroke etiology is important, given that some causes of stroke are associated with etiology-specific additional secondary stroke prevention treatment.
- Treatment for secondary stroke prevention generally is centered on antithrombotic therapy (antiplatelet or anticoagulation when indicated), statins, and risk factor modification (including management of hypertension, hypercholesterolemia, diabetes, and obstructive sleep apnea).
- In the management of atrial fibrillation, novel oral anticoagulants have been shown to be at least noninferior to warfarin for ischemic stroke prevention and better tolerated in terms of intracranial bleeding risk.
- Although symptomatic external carotid artery stenosis is amenable to revascularization, stenting of intracranial atherosclerosis currently is not recommended in the United States.
- Uses of short-term dual antiplatelet therapy (e.g., aspirin plus clopidogrel) include symptomatic severe intracranial atherosclerosis as well as minor stroke and high-risk transient ischemic attack.
Stroke is a common problem, affecting nearly 800,000 people annually in the United States and serving as a leading cause of significant long-term disability.1 Annual stroke costs in the United States alone were estimated recently at $46 billion, with significant concern that these costs are progressively increasing.1,2
This article begins with a brief discussion of stroke epidemiology and then provides an overview of the various stroke mechanisms, setting a framework for which to consider etiology-specific stroke management. Finally, it concludes with a brief discussion on transient ischemic attack (TIA). Throughout the article, there is a focus on current evidence-based practice and, when lacking, usual practice and expert consensus.
Stroke Epidemiology
On average in the United States, someone has a stroke every 40 seconds and someone dies from a stroke every four minutes.1,3 Of stroke survivors, 10% recover nearly completely and, conversely, 10% require care in a long-term care facility.4 Stroke risk increases with age,1,5 although strokes in people ages 18 to 50 years account for roughly 10% to 15% of all strokes.6 Despite encouraging data about declining stroke incidence, the aging population and accumulating risk factors contribute to an increasing lifetime risk of stroke.1 Racial disparities in stroke are significant, with Blacks having a 2.4-fold and Hispanics a 2.0-fold increased incidence of stroke compared with whites living in the same urban community.7 Females also have a higher lifetime risk of stroke than males, particularly in the oldest age groups, and are shown to be less likely to undergo standard diagnostic testing compared to men.1,8
Stroke Mechanisms
Reduced cerebral blood flow that causes damage to neuronal tissue is known as ischemic stroke. The main mechanisms of stroke are thrombosis and embolism of a cerebral artery. Thrombosis refers to a local occlusive process formed in situ within the artery. The site of the obstruction may occur either within a large intra- or extracranial artery (which typically is the result of atherosclerosis) or within a small penetrating artery (which typically is the result of chronic vessel changes from hypertension).9,10 Embolism refers to a thrombus that travels from the site of formation and lodges within distal vessels. The source of the embolism may be either a proximal artery or the heart; paradoxically, the source may be from the venous system in the case of a patent foramen ovale (PFO).9,10 Embolic more so than thrombotic strokes tend to cause symptoms that are abrupt and maximal at onset.11
There have been many attempts to further categorize causes of stroke for clinical and research purposes. A commonly used classification schema for defining stroke subtypes is known as the Trial of Org 10172 in Acute Stroke Treatment (TOAST) system.11 (See Table 1.) The TOAST system has inherent limitations, including the high number of strokes classified as cryptogenic (i.e., having undetermined etiology). Nevertheless, it is a useful tool for conceptualizing our understanding of stroke and for framing mechanism-specific treatment.
Table 1. TOAST Classification System of Stroke Subtypes | |
Stroke Subtype | Prevalence of Stroke Subtype42 |
Large-artery atherosclerosis | 15.5% |
Cardioembolism | 27.8% |
Small vessel occlusion | 18.1% |
Stroke of other determined etiology | 4.2% |
Stroke of undetermined etiology
| 34.4% |
TOAST: Trial of Org 10172 in Acute Stroke Treatment | |
Large-Artery Atherosclerosis
Large-artery atherosclerosis is classically defined as significant stenosis (> 50%) or occlusion of a major brain artery or branch cortical artery, presumably as the result of atherosclerosis.9,12 This refers to both intracranial and extracranial (carotid or vertebral) atherosclerosis.
Intracranial Atherosclerosis. Intracranial atherosclerosis is common in Hispanic, African-American, and, particularly, Asian patient populations (with estimates as high as 50% in specific Asian subpopulations) and likely is the most common cause of stroke worldwide.13 The rate of stroke with cerebral atherosclerotic disease is as high as 15% per year in randomized trials.14,15 This risk is doubled in patients with severe intracranial stenosis (70% to 99%).16 Intracranial atherosclerosis can cause stroke through several mechanisms, primarily in-situ thrombosis with distal “artery-to-artery” embolism and progressive luminal stenosis resulting in a hemodynamically low-flow state. Computed tomography (CT) angiography with contrast or magnetic resonance (MR) angiography without contrast can evaluate noninvasively for intracranial atherosclerosis.
Extracranial Carotid Artery. Carotid disease is found primarily in the internal carotid artery, typically distal to its bifurcation from the common carotid, and can become symptomatic with the rupture of the atherosclerotic plaque with subsequent embolism to the brain hemisphere or eye (presenting as retinal artery occlusion or amaurosis fugax).12 Generally, increased stenosis > 70% compared to 50% to 69% is associated with even greater risk of stroke. However, in some cases, severe stenosis also can lead to formation of collateral supply and, in this manner, can protect against stroke.12,17 There are multiple methods to image carotid stenosis, including CT and MR angiography, as well as ultrasound.
Cardioembolism
Cardioembolism occurs when particulate material from a proximal source travels through the arterial system to lodge within a downstream cerebral artery.
Emboli to the brain most often are composed of mural thrombi or platelet aggregates and typically travel to the anterior circulation (particularly the middle cerebral artery territory), given that these arteries accept the majority of cerebral blood flow.18 Cerebral emboli from a primary cardiac source must be distinguished from cerebral emboli from an alternate source, including a proximal large artery, the venous circulation in the case of a PFO, or unknown (cryptogenic).10 A cardiac (central) source is implied if the emboli result in bilateral infarcts or in both anterior and posterior circulation, particularly if they are cortical and in multiple vascular territories.19 (See Figure 1.) For example, a potential cardioembolic etiology of stroke would be inferred from magnetic resonance imaging (MRI) demonstrating simultaneously occurring left middle cerebral artery and right posterior cerebral artery acute transcortical infarctions. Frequently, these emboli recanalize spontaneously and are not always visualized on subsequent vessel imaging.19
There are many potential sources of cardioembolism.19 (See Table 2.) Whether a stroke can be attributed to a cardioembolic source requires the identification of a known cardiac risk factor for embolic stroke, as well as the exclusion of other etiologies, such as large artery atherosclerosis or lacunar infarct, based on dedicated neuroimaging.9
Figure 1. MRI Displaying Typical Characteristics of a Stroke of Cardioembolic Etiology | |
A | B |
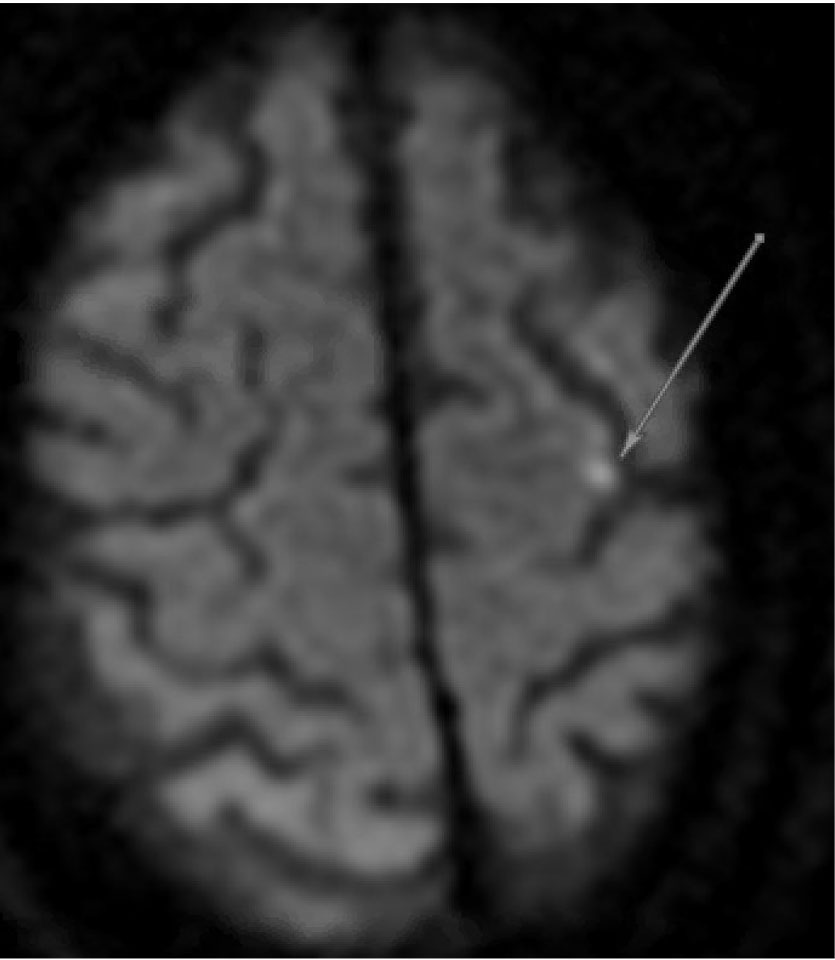 | 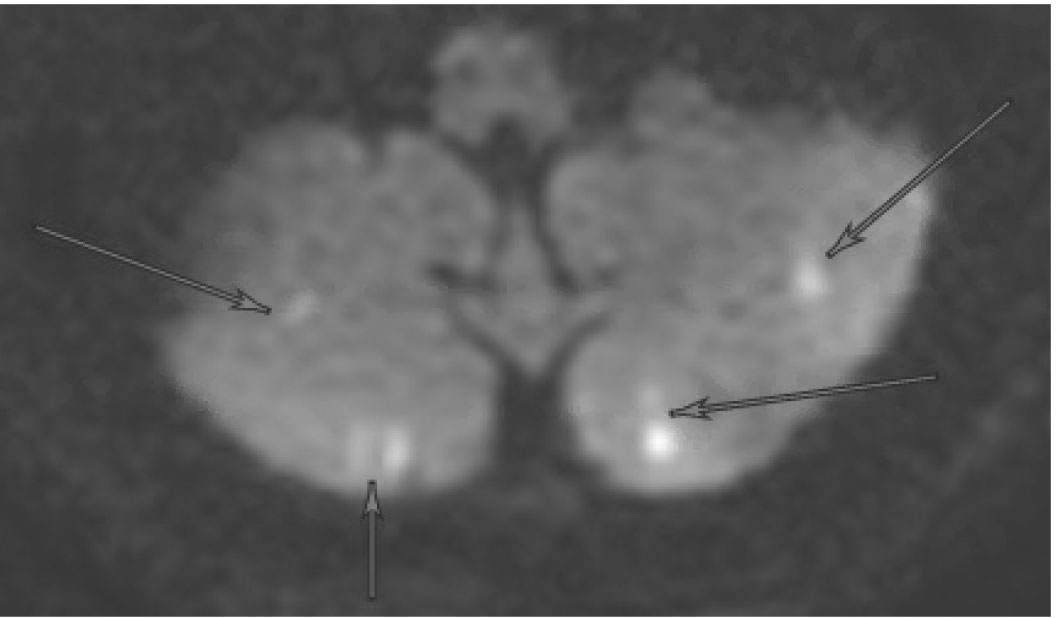 |
DWI sequence demonstrating hyperintensity in the left hemisphere (A) and bilateral cerebellum (B) | |
MRI: magnetic resonance imaging; DWI: diffusion-weighted imaging | |
Table 2. Potential Sources of Cerebral Cardioembolism9 | |
High-Risk Sources | Medium-Risk Sources |
|
|
The most common high-risk sources of cardioembolism include valvular heart disease, endocarditis, the formation of an intracardiac thrombus (e.g., recent myocardial infarction or atrial fibrillation), ventricular or septal aneurysms, and cardiomyopathies. Three specific cardiac sources of stroke (atrial fibrillation, PFO, and aortic arch atheroma) are discussed in depth later. PFO is discussed frequently within the topic of cryptogenic stroke, although for the purposes of our understanding here, we will review it in the context of cardioembolism.
Atrial Fibrillation. Atrial fibrillation is a critical risk factor for stroke. Atrial fibrillation can lead to left atrial or atrial appendage thrombus formation, which can serve as a source of embolism. This arrhythmia is a very strong risk factor for stroke, increasing this risk by four- or five-fold.20 The prevalence of atrial fibrillation has traditionally been described as 1% to 2% of the U.S. population, although, notably, this figure likely is an underestimate because of the degree of undetected subclinical atrial fibrillation, the growing aging population who are at most risk for the development of atrial fibrillation, and the data suggesting widespread global increases of atrial fibrillation over time.20
The presence of atrial fibrillation may be known already at stroke diagnosis, may be discovered during telemetry monitoring during hospitalization, or may be found subclinically on follow-up with outpatient cardiac monitoring. Longer monitoring increases the yield of atrial fibrillation diagnosis.
In the EMBRACE study, the use of an extended cardiac event recorder in cryptogenic stroke patients significantly increased the rate of atrial fibrillation detection compared to standard monitoring at 30 days (16.1% vs. 3.2%).21
Similarly, the CRYSTAL-AF trial demonstrated that the use of an implantable loop recorder further increases the yield of atrial fibrillation detection (8.9% vs. 1.4% at six months, 12.4% vs. 2.0% at one year, and 30% vs. 3.0% at three years).22 The median time from randomization to arrhythmia detection in the CRYSTAL-AF study was 84 days.
Importantly, the pattern of atrial fibrillation (persistent and permanent more so than paroxysmal) has been demonstrated to have a greater association with ischemic stroke,23,24 suggesting that the degree of atrial fibrillation is relevant. It still is unclear to what extent it is clinically relevant to have extended monitoring detect brief paroxysmal atrial fibrillation long after the stroke. This question may be answered in the ongoing NOAH and ARTESiA trials, with expected study completions in 2022.19,25,26
Generally, physicians pursue one month of cardiac monitoring in most patients being evaluated for a suspected cardioembolic stroke (patients with cryptogenic stroke). However, some experts will pursue extended monitoring with implantable devices.
Once identified, stroke risk in atrial fibrillation patients for both primary and secondary stroke prevention can be stratified using scales such as the CHA₂DS₂-VASc score. (See Tables 3 and 4.)
Table 3. The CHA₂DS₂-VASc Score for Atrial Fibrillation | ||
C | Congestive heart failure | 1 point |
H | Hypertension | 1 point |
A₂ |
| 2 points 1 point |
D | Diabetes | 1 point |
S₂ |
| 2 points 1 point |
VASc | History of vascular disease | 1 point |
Table 4. Annual Stroke Risk Stratified by CHA₂DS₂-VASc Score | |
Score | Adjusted Stroke Rate100 |
0 | 0% |
1 | 1.3% |
2 | 2.2% |
3 | 3.2% |
4 | 4.0% |
5 | 6.7% |
6 | 9.8% |
7 | 9.6% |
8 | 6.7% |
9 | 15.2% |
Patent Foramen Ovale. A PFO is a persistent communication between the two atria of the heart, which typically closes after birth. However, in 25% of people, the foramen ovale remains open and could serve as a conduit between the venous and arterial systemic circulations.19 Such a circulatory configuration can lead to the passage of venous thrombotic material into the arterial circulation, potentially resulting in a “paradoxical” embolus to the brain.27,28
PFOs also are debated to potentially cause stroke by other mechanisms, including left atrial dysfunction (particularly in patients with concomitant atrial septal aneurysm), atrial arrhythmia, and clot formation within or on the wall of the PFO.19
A PFO may be detected on echocardiography or transcranial Doppler with the injection of agitated saline, known as a bubble study. Given how common PFO is in the general population, the provider should consider whether the PFO is incidental or relevant before considering PFO-specific treatment, specifically PFO closure, for secondary stroke prevention.29-32
Multiple factors can help assess the likelihood that the PFO is related to the stroke, including the remaining detailed comprehensive stroke workup (including extended cardiac monitoring), the Risk of Paradoxical Embolism (RoPE) score (see Tables 5 and 6), the size and dynamics of the PFO itself (e.g., > 30 microbubbles on echocardiography), and the presence of concurrent venous thromboembolism (VTE).33 Evaluating for concomitant deep vein thrombosis (DVT) is necessary in this context. One may choose to evaluate for a PFO only if it is believed that PFO closure may be considered, generally in patients younger than 60 years of age.
Table 5. RoPE Score | |
Characteristic | Points |
No history of hypertension | 1 |
No history of diabetes | 1 |
No history of ischemic stroke or transient ischemic attack | 1 |
Nonsmoker | 1 |
Cortical infarct on imaging | 1 |
Age (Years) | |
18-29 | 5 |
30-39 | 4 |
40-49 | 3 |
50-59 | 2 |
60-69 | 1 |
70+ | 0 |
RoPE: Risk of Paradoxical Embolism | |
Table 6. Percent of Cryptogenic Strokes Attributable to Patent Foramen Ovale | |
RoPE Score | Percent Attributable |
0 to 3 | 0% (0 to 4) |
4 | 38% (25 to 48) |
5 | 34% (21 to 45) |
6 | 62% (54 to 68) |
7 | 72% (66 to 76) |
8 | 84% (79 to 87) |
9 to 10 | 88% (83 to 91) |
Aortic Arch Atheroma. Aortic arch atheroma is a manifestation of systemic atherosclerosis and can lead to thromboembolism and stroke, particularly in older patients.34-38
Embolism from the aortic arch can occur spontaneously or as a complication of an invasive cardiovascular procedure. Specific aortic arch plaque features described as “complex” plaque pose a greater risk of stroke, including a thickness > 4 mm or ulceration or mobility of a component of the plaque.33,39 Transesophageal echocardiogram is the optimal test to detect complex aortic arch atheromatous disease, with a 90% sensitivity and specificity.33 Transcranial Doppler monitoring can show characteristic high-intensity transient signals (HITS) in patients with complex plaque (as well as other sources of embolism).33 The plaque also can be visualized on CT angiography, MR angiography, transthoracic echocardiogram, and positron emission tomography (PET).11
Before attributing a stroke to this etiology, it is important to rule out other sources of thromboembolism, such as atrial fibrillation, since this finding may necessitate a change in medical management.
Small-Vessel Occlusion
Lacunar stroke refers to the pathophysiological, clinical, and radiographic findings observed in small vessel disease. A lacunar stroke is the result of arterial obstruction of a single deep, penetrating vessel that supplies the subcortical structures of the brain, such as the capsule, basal ganglia, thalamus, and paramedian brainstem.11 Such arterial obstruction is associated with the pathological changes occurring in response to chronic hypertension or diabetes, including microatheroma or lipohyalinosis.11 Lipohyalinosis refers to the degenerative change in small blood vessels caused by the accumulation of lipid within the vessel wall.11
Since the affected arteries are small, the resultant strokes (known as lacunes) also are small. These irregular cavitary lesions typically are < 1.5 cm in diameter.11 Radiographically, lacunar strokes appear as small infarcts in typical subcortical structures, with MRI being more sensitive than CT for detecting these lesions.11 (See Figure 2.) The imaging findings of a small, deep infarct in the absence of other possible stroke etiologies is strongly supportive of a lacunar etiology. Larger areas of infarction, known as giant or super lacunes, may imply thrombosis or embolism of the proximal branch from which the penetrating artery arises; for instance, a lenticulostriate territory stroke could result from a nonocclusive embolus to the middle cerebral artery.11 Therefore, subcortical strokes > 1.5 cm in size may necessitate a diagnostic evaluation aimed at finding an embolic source of stroke, including echocardiography, telemetry, and noninvasive angiography of the head and neck.
Figure 2. MRI Displaying Typical Characteristics of an Acute Stroke of Lacunar Etiology | ||
A | B | C |
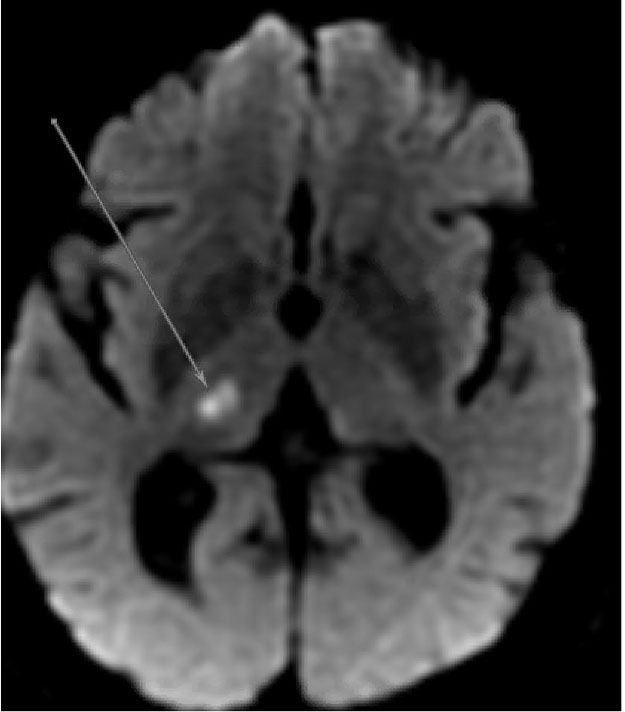 | 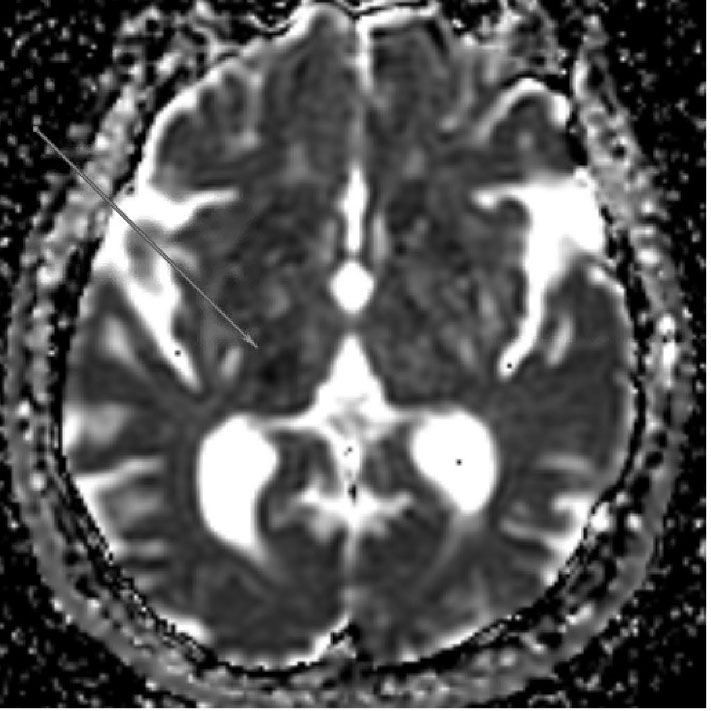 | 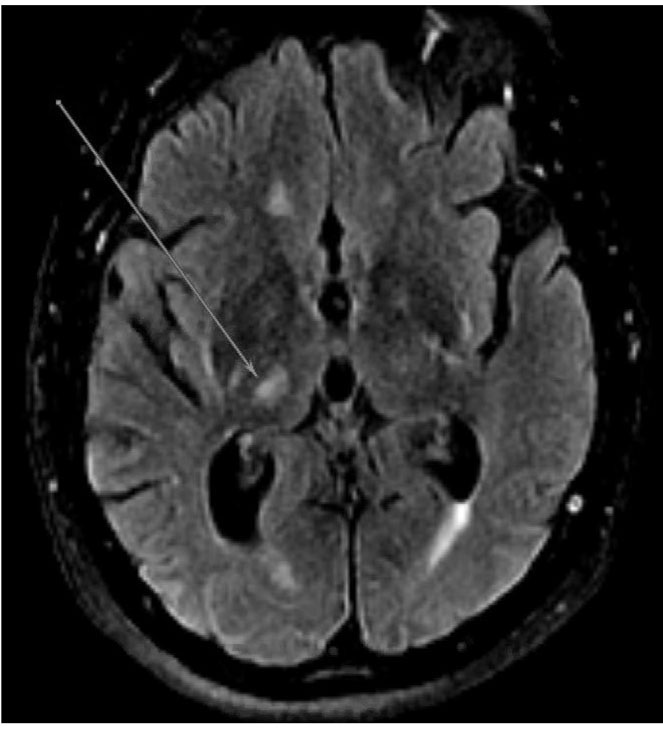 |
DWI sequence demonstrating hyperintensity in the right thalamus consistent with acute/subacute lacunar infarct | ADC sequence demonstrating hypointensity in the right thalamus consistent with acute/subacute lacunar infarct | FLAIR sequence demonstrating hyperintensity in the right thalamus consistent with lacunar infarct; chronic microvascular ischemic changes also are apparent |
MRI: magnetic resonance imaging; DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient; FLAIR: fluid attenuated inversion recovery | ||
Lacunar infarcts clinically present as one of several classic syndromes: pure motor weakness, pure sensory loss, mixed sensorimotor, ataxia hemiparesis, and dysarthria-clumsy hand syndromes.10 These clinical syndromes have moderate sensitivity and specificity for small vessel disease, although there are more rare syndromes still consistent with small vessel disease (including brainstem syndromes and unilateral movement disorders). Additionally, the classic lacunar syndromes alternatively can be caused by strokes of non-small vessel etiology.9 The symptoms of lacunar stroke often fluctuate over the acute course of the disease, hence the name “stuttering lacune.” Although lacunar strokes comprise roughly one-quarter of strokes, they have the lowest in-hospital mortality of the subtypes.40,41
Stroke of Other Determined Etiology
Strokes of other determined etiologies comprise < 5% of stroke subtypes under the TOAST classification system.40,42 There are many specific causes of stroke that do not meet the criteria to be classified as large artery, cardioembolic, or lacunar disease. This includes various rarer causes of stroke, many of which also are more prevalent in younger stroke populations.9 In the stroke literature, “young” typically is defined as younger than age 45 (or 50) years.43 See Table 7 for a list of causes of stroke in the young, which include various nonatherosclerotic angiopathies (e.g., arterial dissection), hematological and genetic conditions (e.g., acquired or congenital hypercoagulable state), and vasculopathy secondary to inflammatory or infectious processes (e.g., primary central nervous system [CNS] vasculitis).44,45 Even in younger age groups, traditional vascular risk factors and stroke mechanisms still may be responsible for an incident stroke, and standard neurovascular imaging and cardiac evaluation should be pursued in young stroke patients. The following section will focus on two specific high-yield etiologies: cervical artery dissection and cancer-related hypercoagulability.
Table 7. Specific Causes and Diagnostic Workup for Rare Etiologies of Stroke in the Young101 |
Infectious Endocarditis: ESR, CBC, TEE, blood cultures Tuberculosis: CSF TB PCR, PPD, chest X-ray Syphilis: RPR, CSF VDRL or FTA-ABS Varicella zoster virus: CSF PCR HIV: Serologic testing Bacterial meningitis: CSF culture |
Autoimmune Primary CNS angiitis: CSF cells and protein, cerebral angiogram, brain biopsy Systemic vasculitis:
Systemic autoimmune diseases: Focused serum rheumatological panel
|
Genetic Factor V Leiden Prothrombin gene G20210A mutation MTHFR C677T mutation CADASIL: NOTCH 3 mutation by skin biopsy Fabry’s disease: alpha galactosidase activity MELAS: Serum and CSF lactate, CK, muscle biopsy, mitochrondrial DNA sequencing Neurofibromatosis Sturge-Weber disease |
Hematologic Protein C/S deficiency Antithrombin III deficiency APLS: lupus anticoagulant, anti-cardiolipin, and anti-beta-2 glycoprotein I antibodies Hyperhomocysteinemia Sickle cell disease: hemoglobin electropheresis DIC TTP Acquired hypercoagulable state:
|
Nonatherosclerotic Angiopathies: Neurovascular Imaging (CTA, MRA, Doppler, DSA)
|
ESR: erythrocyte sedimentation rate; CBC: complete blood count; TEE: transesophageal echocardiogram; CSF: cerebrospinal fluid; TB: tuberculosis; PCR: polymerase chain reaction; PPD: purified protein derivative; RPR: rapid plasma reagin; VDRL: venereal disease research laboratory test; FTA-ABS: fluorescent treponemal antibody absorption; HIV: human immunodeficiency virus; CNS: central nervous system; CTA: computed tomography angiography; MRA: magnetic resonance angiography; PET: positron emission tomography; MTHFR: methylenetetrahydrofolate reductase; CK: creatine kinase; APLS: antiphospholipid syndrome; DIC: disseminated intravascular coagulation; TTP: thrombotic thrombocytopenic purpura; DSA: digital subtraction angiography |
Cervical Arterial Dissection. A dissection results from an intimal tear of the affected artery, allowing blood to extravasate into a false lumen.43,46 This can persist without complication or can lead to stroke caused by intramural hematoma formation and vessel stenosis or as the result of embolization from a thrombus formed at the dissection site.43 Carotid dissection commonly occurs slightly distal to the carotid bifurcation, and vertebral artery dissection most commonly occurs at the C1 to C2 level, where it is most susceptible to injury as it leaves the transverse foramen.43 Classically, dissections are associated with trauma (e.g., direct pressure to or hyperextension of the neck, intense straining, severe coughing) or in patients with connective tissue disorders. However, > 50% of patients with cervical artery dissection are not correlated with a known prior cervical trauma.47
Despite the larger proportion of young patients with stroke from cervical artery dissection, older patients have an absolute number of more cases of cervical arterial dissection strokes, possibly related to increased atherosclerosis weakening the vessel wall.48 Cervical arterial dissection should be considered in patients presenting with ipsilateral neck pain, headache, or a Horner’s syndrome on examination (from disrupted sympathetic fibers running along the carotid artery).
Diagnosis is made with cervical neurovascular imaging; MR and CT angiography are found to have similar sensitivity and specificity in this setting.49,50 MRI with cross-sectional imaging of the vessel can detect intramural hematoma, and contrast angiography can show crescentic enhancement of the vessel or a progressive tapering of the visualized arterial lumen.51
Cancer-Related Hypercoagulability. Cancer-related hypercoagulability is an underappreciated cause of stroke and may be the first sign of an occult cancer.49 About one in 10 hospitalized stroke patients have comorbid cancer.52,53 There are several proposed mechanisms by which cancer is thought to be associated with ischemic stroke.45,54 (See Table 8.) The most common cancers in stroke patients include solid tumors of the lung, gastrointestinal tract, and breast. Clues to occult cancer in ischemic stroke patients or suggestion that the stroke may be secondary to hypercoagulability from cancer include strokes in multiple vascular territories and elevated D-dimer levels.55
Table 8. Ischemic Stroke Mechanisms Specific to Patients with Cancer |
|
Stroke of Undetermined Etiology
The term cryptogenic refers to those strokes of uncertain etiology. Approximately one-third of strokes are classified as cryptogenic.56 Before a stroke is classified as definitely cryptogenic, patients should undergo at least routine diagnostic evaluation for stroke from large artery, cardioembolic, or small vessel disease. The standard workup includes dedicated neurovascular imaging and cardiac testing. Cervical and cranial noninvasive angiography can be accomplished through the use of either MR or contrast CT technologies; transcranial and carotid Dopplers can replace these studies if patients have contraindications to MRI or iodinated contrast. Cardiac evaluation should include a 12-lead electrocardiogram (ECG), inpatient telemetry for at least 24 hours (when acute), and echocardiography. The yield of standard transthoracic echocardiography is rather low for detecting high-risk stroke mechanisms, so if a patient is highly suspected to harbor an occult cardiac source of embolism, transesophageal echocardiography can be performed.56
Other workup can include malignancy screening, hypercoagulable testing, evaluation for a PFO, and occasionally, genetic testing.56 Patients with recurrent strokes of undetermined etiology may benefit from evaluation with a neurologist or stroke neurologist.
The prevailing thought is that a majority of these cryptogenic strokes are from occult embolic sources, hence the recently coined term “embolic stroke of undetermined source” (ESUS). ESUS is defined as a stroke that appears non-lacunar on neuroimaging, without an obvious source after a minimum standard evaluation to rule out known stroke etiologies such as atrial fibrillation and carotid stenosis.55-60 Potential nontraditional embolic etiologies of cryptogenic stroke include covert paroxysmal atrial fibrillation, non-stenosing (< 50%) atherosclerotic disease, and occult hypercoagulable states (e.g., hypercoagulability from occult cancer).57 (See Table 9.)
Table 9. Potential Etiologies of Cryptogenic Stroke |
Covert paroxysmal atrial fibrillation |
Cancer-associated hypercoagulable state, including marantic endocarditis |
Nonstenosing (< 50%) plaques of large cervical or intracranial arteries |
Aortic arch atheroma |
Paradoxical embolism from PFO, atrial septal defect, or pulmonary arteriovenous fistula |
Minor-risk potential cardioembolic sources
|
Branch occlusive disease |
There is increasing emerging evidence that links occult embolic mechanisms to cryptogenic stroke. Cardiac risk factors previously thought to be minor now have been associated with stroke, independent of atrial fibrillation, including supraventricular tachycardia, elevated brain natriuretic peptide (BNP), abnormal P wave morphology, and left atrial dilatation on echocardiography.58-62
Many patients with cryptogenic stroke who undergo prolonged arrhythmia monitoring eventually are discovered to have covert paroxysmal atrial fibrillation.56 Additionally, arterial stenosis < 50% has been associated with ischemic stroke as a source of atheroembolism.63 Many of these etiologies increase the risk for recurrent ischemic events.64
Management
Management of Large Artery Atherosclerosis
Management of Intracranial Atherosclerosis. Intracranial atherosclerotic disease is managed medically with antiplatelet agents and aggressive risk factor control, including blood pressure reduction and statin use.65 The evidence for this recommended practice in the United States comes from several major clinical trials comparing antiplatelets to anticoagulants, and medical therapy to intracranial stenting.66-68
Anticoagulants (e.g., warfarin) or endovascular angioplasty or stenting of intracranial arterial stenosis are harmful and not recommended. Dual antiplatelet therapy with aspirin 325 mg and clopidogrel 75 mg daily for 90 days is recommended for patients with symptomatic intracranial atherosclerosis.
Management of Extracranial Carotid Atherosclerosis. Optimal medical therapy to include an antiplatelet, statin, and risk factor modification is recommended for all patients with carotid stenosis in addition to consideration for revascularization. Carotid stenosis can be revascularized with either carotid endarterectomy (CEA) or carotid artery stenting (CAS) when deemed appropriate. For symptomatic carotid disease, CEA has been shown to be strongly beneficial in patients with 70% to 99% symptomatic stenosis (number needed to treat [NNT] = 6) and weakly beneficial for 50% to 69% symptomatic stenosis in men only (NNT = 22) in a pooled analysis of early landmark NASCET, ECST, and Veterans Affairs (VA) trials.65 More recently, landmark studies CREST and SAPPHIRE examined newer carotid artery stenting compared to the established carotid endarterectomy in select patients with symptomatic carotid disease 50% to 99%, which led to the recommendations to consider CAS in patients who are younger or with a history of medical conditions that greatly increase the risk for surgery (e.g., radiation-induced stenosis, restenosis after CEA).65
For asymptomatic carotid disease, management currently is controversial. A pooled analysis of the early landmark ACAS, ACST, and VA studies demonstrated that CEA did reduce ipsilateral stroke in asymptomatic patients, but the absolute risk reduction was small, with a 3% perioperative stroke or death rate.49,69 Given the improvement in aggressive medical management since these early trials, CREST-2 currently is ongoing to re-evaluate carotid revascularization vs. intensive medical management in asymptomatic high-grade carotid stenosis.49
Management of Cardioembolic Stroke
Certain cardioembolic mechanisms of stroke may require short- or long-term anticoagulation or specific surgical treatments. See Table 10 for a list of high-risk cardioembolic stroke mechanisms that may require dedicated anticoagulation as opposed to antiplatelet agents for secondary stroke prevention. Given the possibility for major hemorrhage with anticoagulation, each patient’s individual risk and benefit profile needs to be reviewed prior to initiating this therapy. We consider the new oral anticoagulants when appropriate (discussed further in “Management of Atrial Fibrillation”). Certain high-risk conditions, such as mechanical prosthetic valve, prompt use with warfarin over the new oral anticoagulants, with higher targeted international normalized ratio (INR) goals (2.5-3.5).65,70 Some patients may require only short-term anticoagulation (e.g., recent anterior wall myocardial infarction or left ventricular thrombus).65
Table 10. Cardioembolic Stroke Mechanisms that May Warrant Anticoagulation |
|
Acute and subacute strokes pose a particularly high risk for hemorrhagic conversion, and anticoagulation usually is delayed initially, pending the size of the stroke and an assessment of the risk of recurrent ischemic event. In contrast, patients with septic embolization from infective endocarditis have a higher than average risk for intracranial hemorrhage with both anticoagulants and antiplatelets, and both usually are avoided unless patients have alternate significant cardiac indications for antiplatelets.71 Some cardioembolic conditions may require specific surgical treatments, such as in the case of the resection of an atrial myxoma.
Management of Atrial Fibrillation. It is well known that anticoagulation reduces the risk of embolic stroke in patients with atrial fibrillation. Patients with CHA₂DS₂-VASc scores of ≥ 2 (and some with a score of 1) are deemed to benefit from therapeutic anticoagulation, given that this is the cutoff at which the benefit of stroke reduction usually exceeds the average risk of major hemorrhage as an adverse event from anticoagulant usage. Scoring systems such as HAS-BLED may help quantify the risk of intracranial hemorrhage on anticoagulation, with a score of ≥ 3 prompting caution when prescribing oral anticoagulation.72 (See Tables 11 and 12.)
Table 11. The HAS-BLED Score102 | ||
H | Hypertension: Systolic blood pressure > 160 mmHg | 1 point |
A | Abnormal renal or liver function | 1 point each |
S | Stroke | 1 point |
B | Prior or predisposition to bleeding | 1 point |
L | Labile international normalized ratios | 1 point |
E | Elderly (age > 65 years) | 1 point |
D | Drug or alcohol use | 1 point each |
Table 12. Annual Risk of Major Hemorrhage Stratified by HAS-BLED Score102 | |
Score | Rate of Major Hemorrhage |
0 | 1.1% |
1 | 1.0% |
2 | 1.9% |
3 | 3.7% |
4 | 8.7% |
5+ | 12.5% |
For patients with nonvalvular atrial fibrillation, novel oral anticoagulants have been shown to be at least noninferior to warfarin for ischemic stroke prevention and better tolerated in terms of intracranial bleeding risk in patients with nonvalvular atrial fibrillation.73-77 The risk of intracranial hemorrhage was lower with every novel oral anticoagulant than with warfarin. Of these newer anticoagulants, dabigatran at a dose of 150 mg twice daily and apixaban 5 mg twice daily were found to be superior to warfarin for ischemic stroke prevention.74,75,77 Apart from apixaban, the newer agents have an increased risk of gastrointestinal bleeding events compared with warfarin when given at non-renally adjusted dosages. Additionally, apixaban was the only novel anticoagulant to have a significant benefit over warfarin for all-cause mortality.74,75 These newer medications all tend to have advantages over warfarin in their ease of use, need for monitoring, and drug-drug interactions.
Management of PFO. PFO closure has been controversial until recently. Previously, results from the CLOSURE I, PC, and RESPECT trials were not statistically significant in reducing stroke recurrence.78,79 However, later results from the 2017 RESPECT extended follow-up, REDUCE, and CLOSE trials (and the 2018 meta-analysis) found that PFO closure was more effective than medical therapy for reducing recurrent stroke rates for select patients, providing evidence for percutaneous PFO closure in patients age ≤ 60 years with a cryptogenic embolic-appearing ischemic stroke with a PFO and no evidence for alternate causes of stroke.79-83
At this time, some of the national and society guidelines regarding PFO management were published before these newer studies, but this updated paradigm is reflected in the 2020 American Academy of Neurology practice advisory.84 New onset atrial fibrillation is the most common adverse effect of PFO device closure. As with all patients with previous stroke, patients with PFO, with or without closure, should be treated with an antithrombotic medication.
Management of Aortic Arch Atheroma. Although aortic arch atheroma is known to be associated with embolism, the best treatment to reduce this risk has not been defined clearly. The only randomized trial to directly compare antithrombotic regimens in this population, the ARCH trial, randomized patients with nondisabling stroke or TIA and ≥ 4 mm aortic arch plaque to dual antiplatelets (low-dose aspirin and clopidogrel 75 mg) or warfarin (INR target range 2-3).85 Although the authors reported a non-significant relative risk reduction with antiplatelet therapy, the study was halted because of low recruitment rates and was underpowered to make any definite clinical claims. The low stroke rates in this trial may have been a result of the improved medical management of patients with atherosclerotic cerebrovascular disease in recent years, including aggressive blood pressure control and statin use.
Management of Lacunar Stroke
Since lacunar strokes often are the result of pathophysiological changes from chronic hypertension, aggressive monitoring and control of this risk factor are integral to the secondary stroke prevention of small vessel disease.
Blood pressure, cholesterol, and other vascular risk factors should be managed aggressively. Blood pressure targets for patients with lacunar strokes were studied specifically in the SPS3 trial, which compared a systolic target of 130 mmHg to 149 mmHg to a target of < 130 mmHg. The study found a nonsignificant rate reduction for all strokes, disabling or fatal strokes, and the composite outcome of myocardial infarction or vascular death with the lower target, as well as a statistically significant rate reduction of intracerebral hemorrhage with infrequent serious adverse events.86 An antiplatelet should be selected for the patient, as discussed earlier. Single antiplatelet therapy as well as short-term dual antiplatelet with POINT/CHANCE protocols are appropriate.
Management of Stroke of Other Determined Etiology
The dedicated treatment for a stroke mechanism of non-traditional etiology varies and is targeted to the specific etiology. Such treatments range from antimicrobial therapy for infectious stroke etiologies to corticosteroids or other immunosuppressive agents for autoimmune diseases; or from anticoagulation for certain congenital or acquired hypercoagulable states to surgical revascularization in patients with moyamoya disease.
Management of Cervical Arterial Dissections. There is no high-level evidence demonstrating superiority of either anticoagulation or antiplatelets for cervical arterial dissection. The only randomized trial studying medical therapy for stroke prevention in dissection patients, the CADISS trial, was a feasibility study and was not powered to be a definitive trial.87 This trial randomized 250 patients with cervical carotid or vertebral dissections to treatment with antiplatelets or anticoagulation. At three months, stroke rates were similar and relatively low in both arms (2% with antiplatelet treatment vs. 1% with anticoagulation). The majority of stroke outcomes occurred early within the follow-up period. Because of a lack of adequate power, no expert consensus has been reached on the preferred treatment for dissection. Current guidelines suggest antithrombotic treatment for at least three to six months.88 Antiplatelet therapy generally is preferred, although some experts select short-term anticoagulation specifically for extracranial cervical dissections with high-risk features.
Management of Cancer-Related Hypercoagulability. Because of the limited studies, the optimal antithrombotic for stroke thought to be related to hypercoagulability from cancer remains unclear. Some experts argue for the use of anticoagulation as a first-line treatment for cancer-associated stroke.89 This approach is in part the result of extrapolation from VTE trials in cancer patients which used low-molecular weight heparins.86,90,91 A new study, CARAVAGGIO, found apixaban to be noninferior to dalteparin (low-molecular weight heparin) for the treatment of cancer-associated VTE without an increased risk of major bleeding, and showed apixaban to be a reasonable choice in these patients, with the limited data for cancer patients with stroke.92 Antiplatelet therapy may be preferred for those with a higher bleeding risk, although it generally is held when platelets are < 50,000. Despite medical management, the median survival following stroke in patients with active cancer was found to be only 84 days, with 34% of these patients experiencing recurrent thromboembolic events.93-96
Management of Cryptogenic Stroke
Given the suspicion that the predominance of ESUS is believed to be cardioembolic, it logically follows that ESUS may be treated best with anticoagulation over antiplatelet therapy to prevent recurrent stroke. However, evidence from randomized controlled trials thus far do not show a clear benefit with this general approach. In the meantime, it is reasonable to treat cryptogenic strokes empirically with antiplatelet therapy. Patients with minor stroke (and high-risk TIA) with cryptogenic etiology would be appropriate for short-term dual antiplatelet therapy as described in the POINT/CHANCE protocols.
Transient Ischemic Attack
TIA is an important entity to diagnose given that > 20% of strokes are thought to be preceded by TIAs.13 TIAs currently are defined as brief episodes of neurologic dysfunction from focal cerebral ischemia not associated with permanent infarction.13 This definition is in contrast to the previously accepted paradigm, which included patients with symptoms that lasted < 24 hours, regardless of evidence of ischemia on imaging.97 The majority of patients without evidence of ischemia on imaging have symptoms that last less than one hour, and some experts advocate for this criterion to be incorporated in a new TIA definition.98 An accepted definition of TIA in current practice is an event with focal neurologic symptoms lasting less than one hour and without evidence of acute infarct on imaging.
Patients who have experienced a TIA require urgent evaluation. When TIA is suspected, it is recommended that the patient be evaluated as soon as possible after the event and undergo MR of the brain (or CT if MR is unavailable), ideally within 24 hours of symptom onset, as well as urgent vessel imaging (with either carotid ultrasound, MR angiography, or CT angiography), and cardiac evaluation with at least an ECG.98 Further cardiac workup with echocardiography (at least transthoracic echocardiogram) and cardiac monitoring then is indicated when the cause of the TIA still is unclear.98
Hospitalization and close observation following the TIA, particularly in the immediate and most high-risk period following the event, has the potential to allow for more rapid and frequent administration of tissue plasminogen activator should a stroke occur.98
The ABCD2 score is a commonly used scale that can help stratify this risk of subsequent stroke. (See Tables 13 and 14.) Although not its original intent, clinicians use the ABCD2 score to aid in the triaging of patients presenting to the emergency department with TIA. Patients presenting within 72 hours of a suspected TIA and with an ABCD2 score of 3 or more typically are admitted to the hospital. This system can be used to help triage patients for expedited inpatient evaluation, as opposed to outpatient follow-up, and reduce hospitalizations.99 The ABCD2 does not help assess for high-risk mechanisms of TIA, such as atrial fibrillation or carotid stenosis, which may require a specific urgent intervention (e.g., anticoagulation or a revascularization procedure). If a patient undergoes outpatient workup, the American Heart Association (AHA) and American Stroke Association (ASA) recommend that the basic stroke evaluation be completed within two days.98
Table 13. ABCD2 Score | |
A | Age > 60 years: 1 point |
B | Blood pressure |
C | Clinical features:
|
D | Diabetes: 1 point |
D | Duration:
|
Table 14. Approximate Risk of Stroke After Transient Ischemic Attack | |||
ABCD2 Score | Two-Day | Seven-Day Stroke Risk | 90-Day |
1-3 (low) | 1% | 1% | 3% |
4-5 (moderate) | 4% | 6% | 10% |
6-7 (high) | 8% | 12% | 18% |
Stroke prevention strategies in TIA patients are identical to those with ischemic stroke and include management of modifiable risk factors in addition to etiology-specific treatment as reviewed earlier in the context of stroke management. TIA secondary stroke management has the additional caveat that select patients with high-risk TIA (in addition to mild strokes) may benefit from short-term dual antiplatelet agents such as aspirin 81 mg and clopidogrel 75 mg daily for the first 21 days.
Conclusion
The field of stroke neurology has continued to evolve with increasing evidence-based prevention strategies. We are learning more about the importance of precise risk factor management, the role of statins for atherosclerotic disease, and the use of targeted antithrombotic therapy based on the underlying mechanism of stroke. Whereas large artery atherosclerotic and small vessel disease respond to antiplatelet agents, anticoagulation has been found to be superior in certain high-risk cardiac conditions, such as atrial fibrillation. The recent expansion of oral anticoagulants to include factor Xa and direct thrombin inhibitors has improved significantly the overall risk/benefit profile of the medical therapy for patients with nonvalvular atrial fibrillation. Surgical options also vary by stroke subtype and mechanism. Although symptomatic cervical carotid stenosis is amenable to revascularization with stenting or endarterectomy, stenting of intracranial atherosclerosis is not current practice in the United States. Patients with unexplained stroke after a standard diagnostic evaluation, particularly if young, should have advanced testing to evaluate for rarer causes of stroke. For cryptogenic strokes, anticoagulation has not yet been proven superior in all patients with suspected embolic source, although randomized controlled trials are ongoing regarding the best management in subsets of these patients.
REFERENCES
The list of references is available online at http://bit.ly/2MLdphv
